INTRODUCTION
Tracheostomy, the creation of a surgical airway via the anterior neck, is a frequently performed procedure in the management of critically ill adults requiring prolonged mechanical ventilation, airway protection, or enhanced secretion clearance. In the United Kingdom, approximately 14,000 adult tracheostomies are performed annually.1 Despite its utility, tracheostomy is associated with significant risks, including airway obstruction, accidental decannulation, and infection—each of which can lead to morbidity or mortality if not promptly identified and managed.
Numerous investigations, including national audits and confidential enquiries, have consistently highlighted deficiencies in hospital-based tracheostomy care, citing avoidable harm and inconsistent clinical practices.2–4 The National Confidential Enquiry into Patient Outcome and Death (NCEPOD) specifically identified systemic failures in staffing, equipment availability, and clinical governance as major contributors to adverse events in tracheostomy patients.4
In response, quality improvement (QI) strategies have been implemented internationally and across the UK to address these challenges. When appropriately designed and executed, QI programs have demonstrated improvements in tracheostomy safety, reduced adverse incidents, and enhanced cost-efficiency.5–7 This study evaluates the impact of a targeted quality improvement initiative implemented during the COVID-19 pandemic in a large UK teaching hospital, with the aim of reducing avoidable harm and establishing sustainable safety processes for adult inpatients with a tracheostomy.
Problem Description
The onset of the COVID-19 pandemic placed unprecedented strain on healthcare systems globally, exacerbating existing challenges in tracheostomy care. In the study hospital there was no dedicated clinical oversight for adult patients with a tracheostomy. This absence of structured governance and coordination led to fragmented care delivery and increased risk to patients. It was estimated that approximately 100 adult tracheostomies were performed annually in this facility, but no centralized database existed to track procedures, outcomes, or follow-up care. During the pandemic, the number of tracheostomies increased significantly to support prolonged ventilation strategies. Patients were frequently transferred between wards due to bed shortages, and many clinical staff were redeployed, often without prior experience or adequate training in tracheostomy management. There were no designated cohort wards for patients with a tracheostomy, and standardized care protocols were lacking. These circumstances highlighted an urgent need to improve clinical oversight, staff competence, and equipment availability. The creation of a dedicated tracheostomy practitioner role presented an opportunity to systematically identify gaps in care and implement targeted improvements aimed at reducing preventable harm.
Available Knowledge
A growing body of evidence underscores the importance of standardized tracheostomy care to ensure patient safety and optimize clinical outcomes. Prior studies have shown that basic interventions—such as ensuring emergency equipment is present at the bedside, maintaining proper tube care, and training staff—can significantly reduce the incidence of airway emergencies and critical incidents.5–7 National frameworks, such as the NCEPOD recommendations and the NHS England quality and safety priorities, emphasize the need for systemic improvements in airway management, including the rationalization of equipment and delivery of multidisciplinary training.4,8 Standardization aligns with broader NHS strategic goals to “keep patients safe” and to “make the most effective use of resources.”8
Despite the recognized benefits, implementing and sustaining improvements in tracheostomy care remains challenging. Tracheostomy management is inherently complex and spans multiple specialties and care settings. The literature increasingly supports the use of quality improvement methodologies—including the use of driver diagrams, logic models, and Plan-Do-Study-Act (PDSA) cycles—to manage such complexity.9–13 These tools enable teams to structure change, iteratively test interventions, and link specific activities to measurable outcomes.
However, critical reviews have identified variability in the fidelity with which these tools are implemented. For example, Taylor et al14 found that fewer than 3% of published QI projects using PDSA cycles fully adhered to methodological guidelines. There is a well-documented tension between the theory of improvement science and its practical application in dynamic healthcare settings.15–17 This underscores the need for real-world studies that transparently report both the successes and limitations of QI interventions in complex, resource-limited environments.
Specific Aims
The overarching aim of this quality improvement initiative was to eliminate avoidable harm in adult in-patients with a tracheostomy tube at a large UK teaching hospital. To guide the intervention, the project adopted an evidence-informed approach to develop a high-level, patient-centered aim that was both aspirational and measurable. The defined project aim was: To achieve 0% incidence of avoidable tracheostomy tube-related harm among adult in-patients during hospitalization.
In alignment with this aim, the initiative sought to accomplish the following specific objectives:
-
Enhance equipment availability and standardization by ensuring that all patients with a tracheostomy have immediate access to essential emergency airway equipment and standardized tracheostomy tubes across designated clinical areas.
-
Improve clinical staff competence and confidence through the development, delivery, and evaluation of a structured, multidisciplinary training program focused on routine and emergency tracheostomy care.
-
Establish systems for care implementation and governance by introducing evidence-based policies, clinical guidelines, and consistent data monitoring processes to support ongoing adherence to national standards and institutional protocols.
These objectives were operationalized using quality improvement methodologies, including driver diagrams and iterative Plan-Do-Study-Act (PDSA) cycles, and evaluated using a combination of process, outcome, and balancing measures. The project was explicitly designed to ensure that change strategies addressed both proximal clinical practices and the broader system-level enablers necessary for sustainable improvement.
METHODS
Context
This quality improvement initiative was implemented in a large tertiary academic teaching hospital in the United Kingdom, serving a local population of 1.9 million and providing regional specialty services for over 3.7 million individuals. The hospital contains 74 adult critical care beds across multiple specialties including surgical, medical, neurology, and cardiac intensive care units. Prior to the intervention, the hospital lacked dedicated oversight of adult tracheostomy care, formal governance structures, or standardized protocols. Patients with a tracheostomy were not cohorted to specific wards, and staff caring for these patients varied in experience and training. Equipment was inconsistently available, with over 600 tracheostomy tubes of 17 different types stored across eight separate locations. A retrospective review of incident reports over an 18-month period revealed 90 tracheostomy-related adverse events, with 39% attributed to staffing issues and 17% to equipment failures.
Interventions
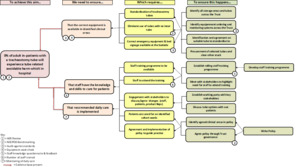
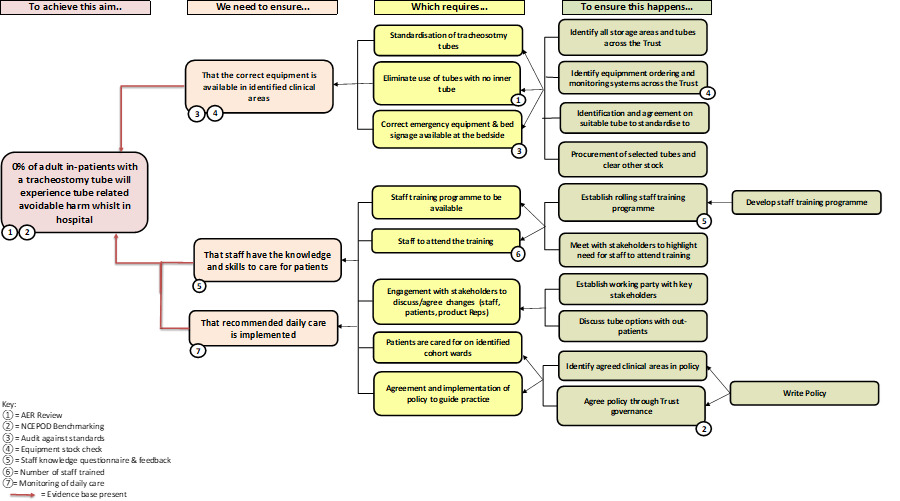
The intervention strategy was guided by the Model for Improvement and was operationalised using a driver diagram to map primary drivers, change ideas, and outcomes. This framework is illustrated in Figure 1.
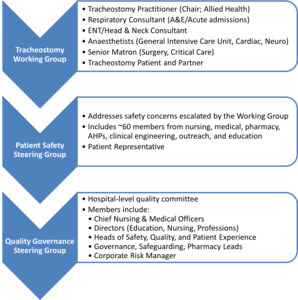
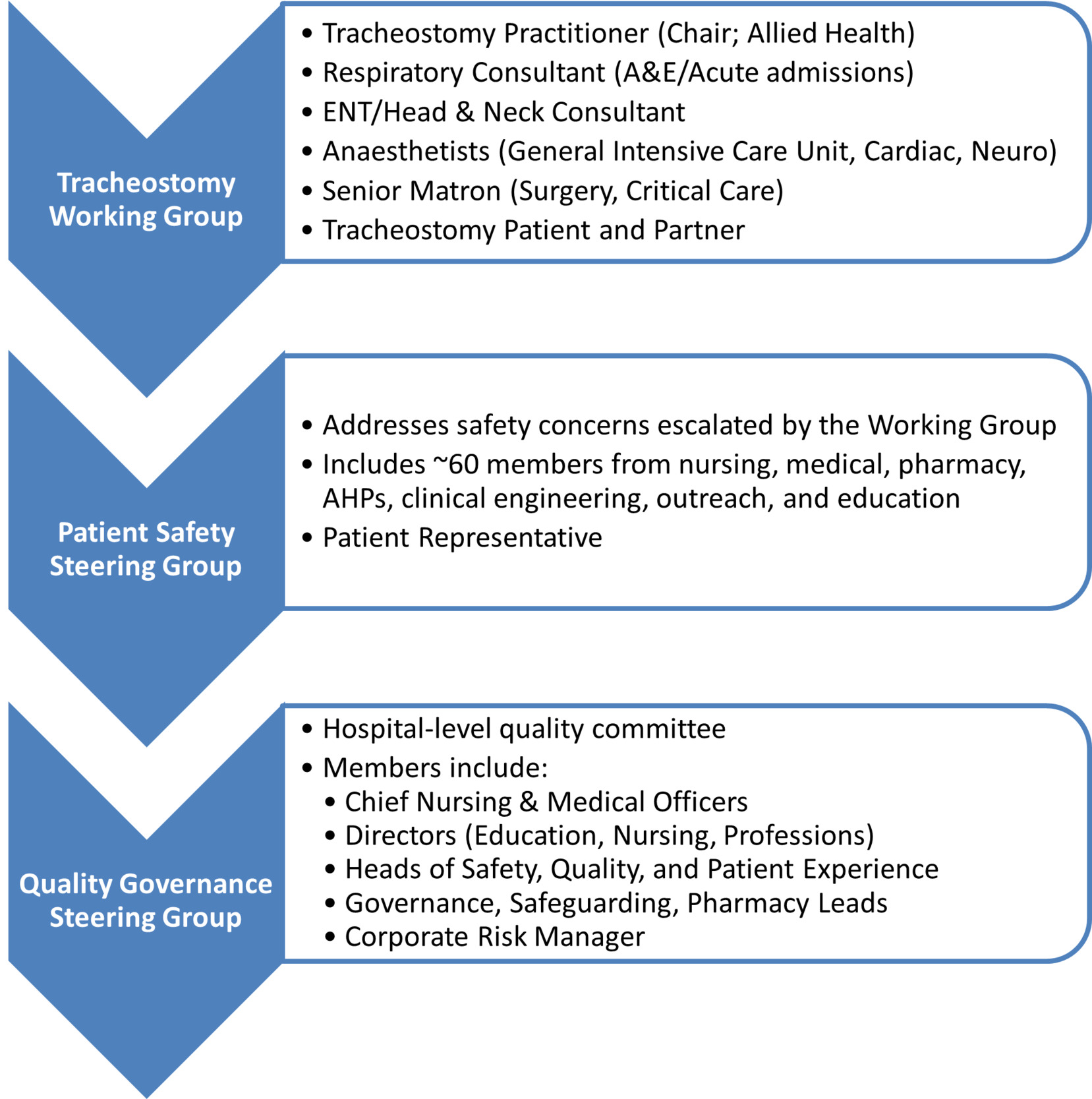
- Stakeholder Engagement: A multidisciplinary working group was established, including representation from speech and language therapy, physiotherapy, critical care, nursing, procurement, and patient advisors. This group co-developed the institutional tracheostomy care policy, clinical care pathways, and education strategies. Stakeholder involvement occurred at multiple levels, and communication pathways were formalised through working groups and governance structures. An overview of stakeholder engagement and discussion forums is shown in Figure 2.
-
Standardization of Equipment: Following a comprehensive stock audit, the number of tracheostomy tube types was reduced, and standardized kits were introduced to clinical areas. A six-month phase-out period was initiated to exhaust existing stock. Emergency equipment bundles and bed signage were also standardized across clinical units.
-
Education and Training: A structured half-day training program was developed, including didactic teaching, skills stations, and simulation-based emergency scenarios. Training content was adapted iteratively using Plan-Do-Study-Act (PDSA) cycles based on staff feedback, attendance data, and pre-post knowledge assessments.
-
Policy and Governance: A comprehensive adult tracheostomy care policy was implemented, incorporating national safety standards, emergency algorithms, and routine care protocols. The policy was disseminated via the hospital intranet and cascaded through clinical governance groups.
Study of the Interventions
To assess the impact of the interventions, a before-and-after study design was employed. Baseline data were collected retrospectively from the 18 months preceding the establishment of the dedicated tracheostomy practitioner role. Post-intervention data were collected prospectively over a 12-month period following implementation. Key measures were tracked at regular intervals, including incident rates, staff training completion, and equipment audit compliance.
PDSA cycles were used to refine intervention components, particularly the education strategy. Feedback was collected via structured post-training evaluations, informal discussions, and process observations.
Measures
Process Measures
-
Percentage of tracheostomy patients with complete emergency equipment at the bedside
-
Staff participation in the training program
-
Compliance with updated tracheostomy care policy and bed signage protocols
Outcome Measures
-
Frequency and severity of tracheostomy-related adverse events (classified using a standard 6-point hospital incident severity scale)
-
Change in staff knowledge and confidence (assessed via pre/post-training questionnaires)
-
Volume and cost of tracheostomy stock in circulation
Balancing Measures
-
Staff time invested in training and equipment reorganization
-
Delays or disruptions to patient care associated with new equipment implementation
Analysis
Descriptive statistics were used to summarize baseline and follow-up data. Frequencies and percentages were reported for categorical variables (e.g. types of incidents, equipment compliance), and pre/post differences in staff knowledge scores were calculated as mean change scores. Due to the quality improvement nature of the project, formal hypothesis testing was not conducted. Severity of clinical incidents was categorized using the hospital’s standard classification system (1 = near miss, 6 = catastrophic), and changes in incident trends were visually tracked using bar charts. Planned time-series analysis of incident data is ongoing and will be incorporated into future evaluations. All results are reported according to the SQUIRE 2.0 guidelines.18
Ethical Considerations
This initiative met criteria for service evaluation and quality improvement under NHS governance policies and did not require research ethics board review. All data collected were de-identified and stored in accordance with institutional data protection protocols. Patient images and videos used in training were obtained with full written and verbal consent. Patient representatives contributed to the working group and were consulted during intervention design and implementation. Staff survey responses were anonymized using the hospital’s learning management system.
RESULTS
Baseline Findings and Problem Identification
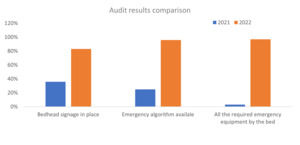
A retrospective baseline assessment conducted over an 18-month period revealed significant safety and system-level deficiencies in tracheostomy care. A total of 90 tracheostomy-related clinical incidents were reported, of which 39% were attributed to staffing limitations (e.g. lack of trained personnel, inappropriate care settings), 17% to equipment availability issues, 12% to blocked tracheostomy tubes, and 11% to accidental decannulations or dislodgements. Equipment audits conducted across wards revealed that only 1 out of 40 patients (2.5%) had the recommended emergency tracheostomy equipment available at the bedside, highlighting a systemic risk to patient safety. The most common themes identified from the 90 incident reports are summarized in Table 1. No central database existed to track the number of tracheostomies performed or the associated outcomes, and training was sporadic and undocumented. The severity of clinical incidents decreased post-intervention, with all incidents classified as “no harm” or “near miss.” Comparative adverse event data before and after intervention are displayed in Figure 3.
Implementation and Evolution of Interventions
The quality improvement initiative was implemented over a 12-month period and included several iterative changes informed by stakeholder feedback and real-time observation. Initial stakeholder meetings led to the development of a driver diagram and consensus on key areas for intervention: equipment availability, staff capability, and care standardization. The equipment rationalization strategy was launched after a comprehensive audit revealed more than 600 tracheostomy tubes across 17 types stored in eight different locations, with an estimated value of £40,000. A phased withdrawal plan was implemented to allow for safe transition to standardised equipment. The initial staff education approach—brief drop-in training—was ineffective, with low attendance and minimal improvement in staff knowledge. This prompted the development of a structured half-day training model that included didactic content, hands-on skill stations, and simulation-based emergency scenarios. Three Plan-Do-Study-Act (PDSA) cycles were used to refine training format, content sequencing, and evaluation strategies. Details of each PDSA cycle and the associated findings are presented in Table 2.
Process Measures: Training, Equipment, and Policy Adoption
Training participation increased from zero at baseline to 187 staff members completing the revised program within 12 months. Pre/post knowledge assessments demonstrated an average increase of 2.8 points on a 10-point scale, with the largest gains observed in emergency response scenarios. All participants (100%) reported increased confidence following the training. Changes to the evaluation protocol—such as requiring post-training survey completion to receive a certificate—improved questionnaire response rates by 42%. Emergency equipment compliance at the bedside increased from 2.5% at baseline to 97.5% post-intervention across all clinical units. Audit results comparing pre- and post-intervention compliance are shown in Figure 4. In parallel, a comprehensive tracheostomy care policy was ratified and disseminated across the hospital. It included algorithms for emergency management, standardized bed signage, and care planning templates. Compliance was monitored through spot audits and team leads were designated in each clinical area to support policy uptake.
Outcome Measures: Patient Safety and Cost Efficiency
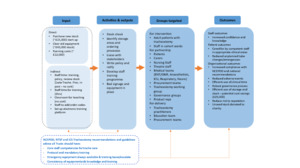
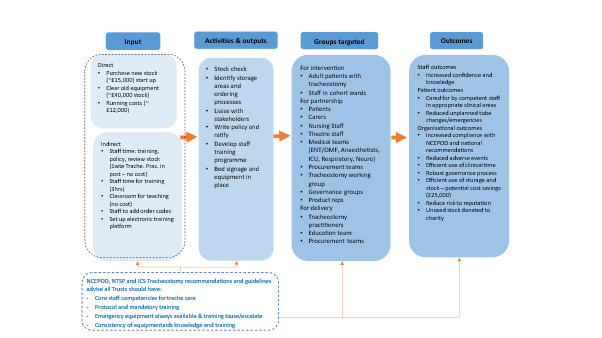
Following intervention implementation, no tracheostomy-related clinical incidents resulted in moderate, severe, or catastrophic harm. All recorded incidents were rated as “no harm” or “near miss,” representing a marked improvement in safety performance. Incident reduction aligned temporally with the rollout of staff training and equipment standardization, suggesting a strong association between these interventions and improved clinical outcomes. Financially, the rationalization of equipment led to significant cost efficiencies. Approximately £40,000 in overstocked tubes were removed from circulation, repurposed for training, or donated. Ongoing procurement was streamlined to include fewer tube types, with projected annual savings of £25,000. A summary of direct and indirect costs and anticipated cost savings is provided in Table 3. The anticipated annual cost of tracheostomy tube procurement was reduced to £12,000 based on usage patterns and procedure frequency. To understand how interventions translated into improved safety and efficiency, a pathways-to-outcomes model was developed. This model is depicted in Figure 5.
Contextual Factors Influencing Implementation
Several contextual variables influenced the intervention’s trajectory and success. The COVID-19 pandemic led to widespread staff redeployment, resulting in care being delivered by individuals unfamiliar with tracheostomy management. Additionally, the absence of designated cohort wards meant patients with a tracheostomy were cared for across disparate clinical areas, complicating standardisation efforts. Despite these challenges, institutional support for patient safety initiatives and alignment with NHS England’s harm-reduction priorities facilitated policy adoption and training integration.
Unintended Consequences and Emergent Benefits
The initiative yielded several unintended benefits. Due to the success and high demand for training, the program was expanded to include laryngectomy care and other healthcare disciplines such as paramedics. Plans are underway to offer the course externally as a revenue-generating activity, with estimated annual income of £7,500. Challenges included variability in stock replenishment systems across wards, which led to uneven distribution of standardised tubes and, in some cases, delayed outpatient appointments. In addition, global supply chain disruptions—linked to COVID-19, Brexit, and geopolitical instability—temporarily affected availability of key tracheostomy supplies, prompting contingency planning.
Missing Data and Measurement Challenges
Some limitations were encountered in data collection and measurement. Early training sessions had low post-session survey completion rates, limiting the completeness of outcome assessment. While this was addressed in later cycles, the early data remain incomplete. Furthermore, due to non-standardised item coding and mixed procurement channels, baseline data on historical tracheostomy tube expenditures could not be accurately reconstructed. Finally, although improvements in adverse events were documented via pre/post comparisons, continuous data tracking using time-series analysis was not available during the initial evaluation. A run chart methodology has since been implemented to support ongoing monitoring.
DISCUSSION
Summary
This quality improvement initiative aimed to reduce avoidable harm among adult in-patients with tracheostomy tubes through a multifaceted intervention encompassing staff training, equipment standardization, and enhanced governance. The key findings indicate a marked improvement in safety outcomes, evidenced by the elimination of moderate-to-severe adverse events and an increase in equipment compliance from 2.5% to 97.5%. Staff knowledge and confidence in tracheostomy care also improved significantly, and financial efficiencies were achieved through stock rationalization. These outcomes align closely with the project’s specific aim: to eliminate avoidable tracheostomy-related harm. A key strength of the initiative was its implementation in a complex clinical environment without additional external funding, relying instead on system-level redesign and cross-disciplinary collaboration.
Interpretation
The association between the interventions and observed outcomes appears strong and temporally aligned. Improvements in adverse event severity and frequency coincided with the rollout of structured training and the introduction of standardized equipment bundles. While causality cannot be definitively established due to the absence of a control group, the use of process measures, iterative PDSA cycles, and triangulated stakeholder feedback support a meaningful relationship between interventions and outcomes.
These findings are consistent with existing literature, particularly multicenter studies from the Global Tracheostomy Collaborative and UK-based NHS initiatives, which have demonstrated improvements in patient safety through standardization, multidisciplinary training, and stakeholder engagement.5–7 However, this project adds to the literature by showcasing what can be achieved in a single institution with constrained resources and limited infrastructure at the outset. The project had both direct and systemic impacts. For patients, the most tangible benefit was the reduction in preventable clinical harm. For the organization, the initiative fostered interdisciplinary collaboration, improved equipment procurement efficiency, and created a replicable model for other high-risk patient populations. Importantly, contextual factors such as pandemic-driven staff redeployment and non-standard procurement systems initially posed barriers but were navigated through adaptive leadership and inclusive engagement strategies.
Unanticipated benefits, including expansion of training to additional disciplines (e.g., paramedics) and emerging revenue opportunities, reflect the potential for broader institutional gains. However, trade-offs were also observed. For example, inconsistencies in stock management led to outpatient appointment disruptions, and global supply shortages delayed full equipment standardization. These strategic tensions highlight the importance of system readiness and supply chain stability when introducing high-reliability interventions.
Limitations
Several limitations should be acknowledged. First, the generalizability of the findings may be limited due to the single-site nature of the project and the unique pressures of the COVID-19 context, which shaped staffing, patient flow, and resource allocation. Additionally, the before-and-after design without a concurrent control group limits the ability to infer causality. Internal validity may have been influenced by measurement inconsistencies—particularly during early phases of data collection, when training surveys were returned inconsistently and incident tracking relied on two-point comparisons rather than run charts. Efforts were made to mitigate these limitations. Survey response protocols were revised mid-cycle to ensure more complete outcome data, and a time-series charting approach has now been adopted for prospective incident monitoring. The use of triangulated data sources (incident reports, audit data, stakeholder feedback) further strengthens the reliability of observed trends. Nonetheless, opportunities remain to improve fidelity in implementation science methodology—particularly in the real-time analysis of process control data and in capturing patient-reported outcomes.
Conclusions
This project demonstrates that significant improvements in the safety and quality of tracheostomy care are achievable through locally driven, resource-conscious interventions guided by improvement science principles. The work has shown clear utility for improving patient outcomes, optimizing clinical workflow, and reducing unnecessary costs. Moreover, the iterative development of the tracheostomy training program and governance structures has supported sustainability, with institutional integration now in place for ongoing monitoring and staff education. The potential for spread is considerable. The methods, logic model, and implementation strategies may be adapted to similar high-risk clinical processes, particularly in institutions with fragmented care pathways or resource constraints. Future directions include greater incorporation of patient voice, co-production of care pathways, and integration of digital tools such as virtual simulation or real-time dashboards for quality monitoring. In summary, this initiative reinforces the value of structured, evidence-informed improvement efforts in advancing patient safety and system reliability—even within highly complex, resource-limited healthcare settings.
ACKNOWLEDGEMENTS
The authors acknowledge the guidance and expertise of Dr. Susan Procter, Dr. Chih Hoong Sin, and Mr. Mike Davidge of Buckinghamshire New University, as well as Ms. Tracey Marriott and Mr. Ferdinand Manansala from the Oxford Academic Health Science Network, whose instruction through the Adopting Innovation and Managing Change in Healthcare Settings program provided critical conceptual and practical foundations for this quality improvement initiative.
The authors also extend their sincere appreciation to the multidisciplinary staff of University Hospital Southampton NHS Foundation Trust for their continued commitment to improving tracheostomy care, and to the patients and families whose engagement and feedback meaningfully informed the development and implementation of this project.