INTRODUCTION
Each year, more than 795,000 people in the United States experience a stroke, approximately 87% of which are ischemic in nature.1 Among these patients an estimated 50% require tracheostomy to manage severe neurological impairment and airway compromise.2 As hyperacute stroke therapeutics advance and include patients with more severe stroke, tracheostomy utilization is steadily rising, reflecting its role in enhancing airway management through earlier liberation from the ventilator in critically ill patients.3 Prior randomized trials studying early versus late tracheostomy placement after stroke suggested that it is feasible but longer term outcomes were equivocal. Thus, the timing of tracheostomy placement varies widely, with decisions often relying on subjective clinician judgment rather than evidence-based practices.4–10 This lack of standardization could contribute to unnecessary surgical procedures, prolong hospital stay, and delay access to early rehabilitation, potentially impacting long-term recovery after stroke.10
No consensus exists on optimal timing of tracheostomy in neurocritical patients. Although early tracheostomy (<14 days) has been associated with reduced aspiration risk, lower incidence of ventilator-associated pneumonia (VAP), expedited discharge from intensive care unit (ICU), and improved rehabilitation trajectories, tracheostomy carries risk of anesthesia and procedural, or postoperative adverse events.10–13 Existing evidence in the stroke population presents mixed findings, with some meta-analyses supporting early intervention and others showing no significant differences in length of stay (LOS) or mortality.14–17 This inconsistency complicates clinical decision-making and emphasizes the need for targeted research addressing timing of tracheostomy in neurocritical patients.18
We sought to understand timing of tracheostomy placement and association with relevant clinical outcomes in cohorts of stroke and non-stroke patients admitted to a specialty neurocritical care unit in an urban, quaternary care hospital. By examining ICU LOS, ventilator dependence, complications, and discharge disposition, this work aims to inform current practices on timing of tracheostomy care. Insights into outcomes of early versus late tracheostomy could support evidence-based decision-making, reduce practice variability, and facilitate tailored interventions that improve recovery trajectories for critically ill patients.
METHODS
This study adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational research.19
Study Design, Setting, and Participants
This retrospective cohort study involved the analysis of prospectively collected data using a Microsoft Access database. The study was conducted in a neuro-ICU at Johns Hopkins Medicine and was approved by the institutional review board (IRB00081292). The cohort included adult patients (≥18 years old) with neurological injury, admitted between April 2007 and December 2013 who underwent both percutaneous tracheostomy and gastrostomy during their hospitalization. This is a secondary data analysis of previously collected data. Participants were prospectively identified at the time of tracheostomy placement during their hospitalization. Identification was done in real-time by the clinical or research team, who were notified of upcoming tracheostomy procedures through daily ICU rounds, operative schedules, or direct communication with the critical care and surgical teams. Patients were excluded if they had a tracheostomy or gastrostomy tube prior to admission or if they did not undergo gastrostomy. Patients with pre-existing tracheostomy or gastrostomy were excluded to ensure a standardized cohort undergoing tracheostomy during hospitalization, minimizing confounding from prior airway or nutritional support. Patients without gastrostomy were also excluded to enhance comparability, as concurrent placement often indicates prolonged ventilation and a similar clinical trajectory.
Variables, Data Sources, and Measurement
Demographic variables collected for this study included stroke status (stroke vs. non-stroke), age, sex, race/ethnicity, body mass index, calculated as weight in kilograms divided by height in meters squared), age-adjusted Charlson Comorbidity Index (CCI),20,21 and admission Glasgow Coma Scale (GCS) score.22–24 These variables provided a baseline characterization of the study population.
Hospital course metrics were analyzed to evaluate the association of tracheostomy timing with relevant patient outcomes. These metrics included time to events such as liberation from the ventilator, total cost of stay, reintubation occurrences prior to tracheostomy, tracheostomy-related complications, and LOS in the hospital, neuroscience ICU, and post-tracheostomy periods. Complications were categorized into healthcare-associated infections, such as aspiration pneumonia and tracheitis, stomal infections, or other procedure-related adverse events.
LOS metrics were calculated to assess the temporal relationship between tracheostomy timing and hospitalization duration. Hospital LOS was defined as the duration from the day of admission to discharge, while ICU LOS was calculated from the day of ICU admission to discharge. Time to tracheostomy was measured as the number of days from admission to the procedure. Post-tracheostomy LOS was defined as the duration between the tracheostomy procedure and hospital discharge. Tracheostomy timing was classified into two categories: early and late. Early and late tracheostomy were defined as occurring within the first 14 days of admission or later than 14 days after admission, respectively, based on recent literature.5–8 This classification was used to examine differences in clinical outcomes between these two groups.
Statistical Methods
Descriptive statistics were used to summarize patient characteristics and outcomes. Categorical variables were reported as frequencies and percentages, while continuous variables were presented as means with standard deviations for parametric data or medians with interquartile ranges for non-parametric data. The Kolmogorov-Smirnov test was applied to assess the normality of continuous variables.
For unadjusted comparisons, Student’s t-test or the Mann–Whitney U test was used for continuous variables, depending on their distribution. Categorical variables were compared using the chi-square test or Fisher’s exact test, as appropriate. Multivariate analysis was employed to examine adjusted associations between stroke status, tracheostomy timing, and clinical outcomes such as costs and ventilator days. Multiple linear regression models were used for continuous outcomes, while logistic regression models were used to assess associations with binary outcomes, including tracheostomy-related complications and reintubation.
Time-to-event outcomes, such as discharge to rehabilitation or death, were analyzed using Fine and Gray competing risks models.25 These models accounted for competing events, including death, hospice transfer, and interhospital transfer. Sub-hazard ratios (SHR) and their corresponding 95% confidence intervals (CI) were calculated to evaluate the cumulative incidence of events.26 To enhance model validity, covariate adjustments were made for age, sex, race, admission GCS score, hospital LOS, and ICU LOS. Multicollinearity among covariates was assessed using variance inflation factors, with values below 3 confirming the absence of significant multicollinearity.
Data Handling and Missing Data
Data handling and validation procedures were implemented to ensure the integrity of the dataset. Missing values were addressed using appropriate imputation methods. One missing cost value was imputed by calculating the product of the average cost per hospital day for demographically similar patients and the total hospital LOS. For two patients with unclear ICU LOS, values were imputed using the mean ICU fraction of the total LOS for patients with comparable tracheostomy indications and discharge dispositions.
Statistical Software
All statistical analyses were conducted using R version 4.4.1 (R Foundation for Statistical Computing, Vienna, Austria). A two-tailed p-value of <0.05 was considered statistically significant.
RESULTS
Participant Selection
Retrospective review identified 371 patients who underwent tracheostomy during the study period, of whom 290 met inclusion criteria. Equal numbers of patients had stroke and non-stroke neurological diagnoses (Figure 1). Primary stroke subtypes, classified based on neuroradiologist interpretation of magnetic resonance imaging, included intracerebral hemorrhage (44.1%), subarachnoid hemorrhage (26.9%), and ischemic stroke (26.2%). Primary non-stroke diagnoses included elective nonvascular, post-surgical (22.8%), traumatic brain injury (18.6%), encephalopathy (15.2%) and other diagnoses (24.8%). Indications for tracheostomy included chronic ventilator dependence (84.4%), airway protection due to mental status (12.1%), inability to wean (3.1%), and aspiration risk (1.4%).
STROKE VS. NON-STROKE COMPARISONS
Characteristics Comparing Non-Stroke and Stroke Patients
The study included 290 patients, with 145 in each group (Table 1). Stroke patients were older than non-stroke patients (59.3 ± 13.6 vs. 53.0 ± 17.8 years, p < 0.001), and had a higher proportion of females (58.6% vs. 46.2%, p = 0.045) and Black patients (47.6% vs. 29.7%, p = 0.016). There were no significant differences in BMI (31.0 ± 10.1 vs. 28.0 ± 9.7, p = 0.086) or age-adjusted CCI (2.4 ± 2.5 vs. 2.4 ± 2.8, p = 0.562). Stroke patients had lower GCS scores on admission (8.3 ± 3.6 vs. 9.9 ± 4.2, p = 0.001). Time to tracheostomy was similar between groups (14.6 ± 7.1 vs. 15.2 ± 9.5 days, p = 0.968).
Outcomes Comparing Stroke and Non-Stroke Patients (Unadjusted)
Among the 290 patients included in the study (non-stroke: n = 145; stroke: n = 145), significant differences were observed in several clinical outcomes between the groups (Table 2). Non-stroke patients had a significantly higher rate of reintubation compared to stroke patients (35.9% vs. 23.4%, p = 0.03). ICU LOS was longer for non-stroke patients (28.8 ± 15.4 vs. 24.5 ± 10.7 days, p = 0.03), as was the time from tracheostomy to discharge (26.7 ± 20.8 vs. 20.7 ± 14.5 days, p < 0.01). While total hospital LOS trended longer in non-stroke patients, this difference did not reach statistical significance (p = 0.07). Additionally, hospitalization costs were significantly higher in non-stroke patients compared to those with stroke ($167,219 ± 88,411 vs. $138,113 ± 60,333, p < 0.01). Tracheostomy-related complications and discharge disposition did not differ significantly between groups.
Outcomes, Comparing Stroke and Non-Stroke Patients (Adjusted)
Predictors of Reintubation Prior to Tracheostomy in Stroke and Non-Stroke Patients
In adjusted regression analyses, reintubation prior to tracheostomy was significantly associated with multiple factors (Table 3). Among all patients, age in years (OR: 1.03, 95% CI: 1.01–1.05, p < 0.01) and admission GCS scores (OR: 1.14, 95% CI: 1.06–1.23, p < 0.01) were linked to increased odds of reintubation. Female sex was associated with lower odds of reintubation in the overall cohort (OR: 0.80, 95% CI: 0.46–1.37, p = 0.03) and in stroke patients (OR: 0.43, 95% CI: 0.19–0.98, p = 0.04), but not in non-stroke patients. Additionally, stroke patients had a significantly lower likelihood of reintubation compared to non-stroke patients (OR: 0.54, 95% CI: 0.31–0.95, p = 0.03).
In subgroup analyses, non-stroke patients demonstrated a significant association between higher admission GCS scores and increased odds of reintubation (OR: 1.15, 95% CI: 1.05–1.26, p < 0.01). Among stroke patients, late tracheostomy was significantly associated with higher odds of reintubation (OR: 2.71, 95% CI: 1.15–6.39, p = 0.02).
Predictors of Discharge to Acute/Chronic Rehabilitation and Death or Discharge to Hospice
In adjusted time-to-event analyses accounting for age, sex, race, and admission GCS scores, stroke was significantly associated with an increased likelihood of discharge to rehabilitation following an ICU stay (SHR: 1.41, 95% CI: 1.08–1.84, p = 0.01) (Table S1). Additionally, stroke patients had a higher risk of death or discharge to hospice during their ICU stay (SHR: 2.22, 95% CI: 1.07–4.62, p = 0.03) (Table S2).
Predictors of Total Hospital Costs in Stroke and Non-Stroke Patients
In the analysis of total hospital costs among all patients, stroke patients, and non-stroke patients, hospital LOS and ICU LOS emerged as the strongest predictors of increased hospital costs across all groups (Table 4). For the entire cohort, hospital LOS (β = 0.606, p < 0.01) and ICU LOS (β = 0.385, p < 0.01) were significantly associated with higher hospital costs. Among stroke patients, hospital LOS (β = 0.535, p < 0.01) and ICU LOS (β = 0.419, p < 0.01) remained the most significant predictors of higher costs. Additionally, female sex was associated with significantly increased hospital costs (β = 0.093, p = 0.02). For non-stroke patients, hospital LOS (β = 0.651, p < 0.01) and ICU LOS (β = 0.356, p < 0.01) were again the strongest predictors of higher total hospital costs. The regression models demonstrated strong predictive ability, with adjusted R-squared values of 0.85 for all patients, 0.79 for stroke patients, and 0.87 for non-stroke patients. The models were statistically significant (p < 0.001) across all groups.
EARLY VS. LATE TRACHEOSTOMY
Patient Characteristics Comparing Early vs. Late Tracheostomy
In comparing stroke patients who underwent early versus late tracheostomy, there were no significant differences in baseline characteristics, including age, sex, race, body mass index, Charlson comorbidity index, and admission GCS score (Table 5). Among non-stroke patients, those who underwent late tracheostomy were significantly older than those who received early tracheostomy (57.3 ± 17.3 vs. 48.7 ± 17.4 years, p < 0.01) (Table 6). Additionally, the age-adjusted CCI was higher in the late tracheostomy group (2.9 ± 2.9 vs. 1.9 ± 2.6, p = 0.03), indicating a greater burden of comorbid conditions.
Primary Outcomes Comparing Early vs. Late Tracheostomy (Unadjusted)
In stroke patients, early tracheostomy was associated with significantly higher tracheostomy-related complications compared to late tracheostomy (22.1% vs. 7.3%, p = 0.03) (Table 7). However, patients who underwent late tracheostomy had significantly higher rates of reintubation (33.8% vs. 14.3%, p < 0.01) and longer ICU LOS (27.6 ± 9.2 vs. 21.7 ± 11.2 days, p < 0.01), hospital LOS (38.9 ± 15.1 vs. 32.4 ± 13.7 days, p < 0.01), and total hospital costs ($157,304 ± 59,118 vs. $121,645 ± 56,406, p < 0.01). Interestingly, patients in the early tracheostomy group had a longer tracheostomy-to-discharge time (22.5 ± 13.7 vs. 18.7 ± 15.2 days, p = 0.02), but there were no significant differences in discharge location between the two groups (p = 0.63).
Among non-stroke patients, late tracheostomy was associated with significantly worse outcomes compared to early tracheostomy (Table 8). Patients in the late tracheostomy group had a longer ICU stay (35.5 ± 14.5 vs. 22.1 ± 13.2 days, p < 0.01) and prolonged LOS (50.8 ± 22.6 vs. 32 ± 18 days, p < 0.01). Additionally, total hospital costs were significantly higher in the late tracheostomy group ($206,184 ± 89,984 vs. $128,788 ± 68,063, p < 0.01). Although reintubation rates were higher in the late tracheostomy group (43.1% vs. 28.8%), this difference did not reach statistical significance (p = 0.07).
Impact of Timing on Discharge to Acute/Chronic Rehabilitation
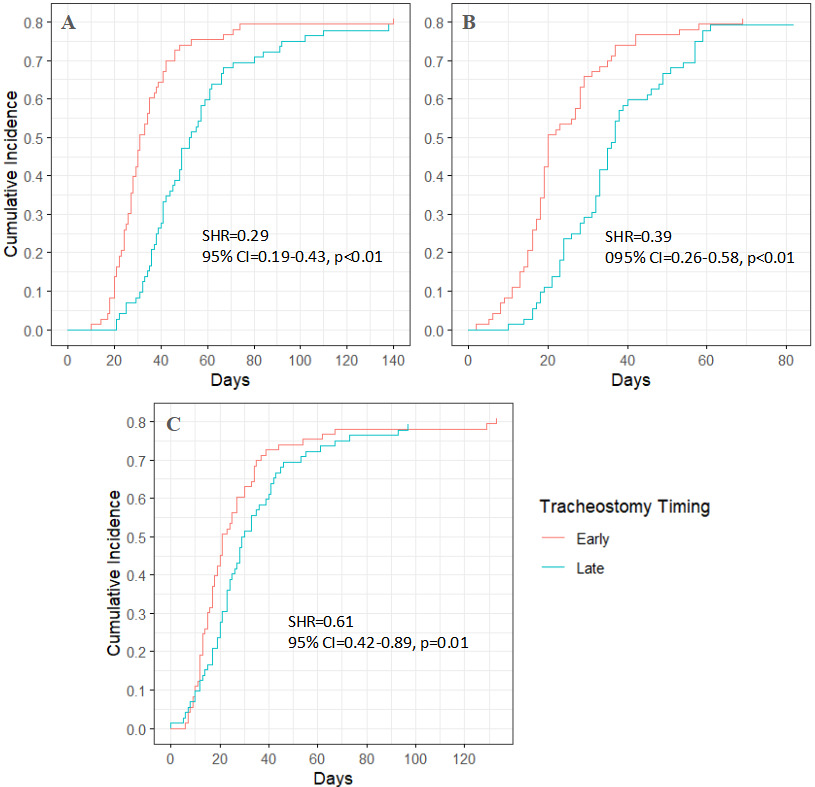
Among stroke patients, the median time to discharge to rehabilitation was 31 days from hospital admission and 19 days from ICU admission for those who underwent early tracheostomy. In contrast, for those who received late tracheostomy, the median times were 38 days from hospital admission and 28 days from ICU admission (Figures 2A–B). Post-tracheostomy, the median time to discharge to rehabilitation was 21 days in the early tracheostomy group and 17 days in the late tracheostomy group (Figure 2C). Patients who underwent late tracheostomy had a 40% lower likelihood of discharge to rehabilitation from hospital admission (SHR = 0.60, p < 0.01) and from ICU admission (SHR = 0.56, p < 0.01) (Table S3).
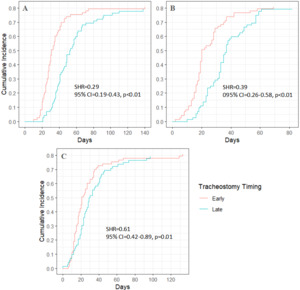
Among non-stroke patients, the median time to discharge to rehabilitation was 30 days from hospital admission and 20 days from ICU admission for those who underwent early tracheostomy. In contrast, for those who received late tracheostomy, the median times were 49 days from hospital admission and 35 days from ICU admission (Figures 3A–B). Post-tracheostomy, the median time to discharge to rehabilitation was 21 days in the early tracheostomy group and 28 days in the late tracheostomy group (Figure 3C). The association between late tracheostomy and decreased likelihood of discharge to rehabilitation was even stronger in non-stroke patients, with a 71% lower likelihood (SHR = 0.29, p < 0.01) from hospital admission and a 61% lower likelihood (SHR = 0.39, p < 0.01) from ICU admission (Table S4).
Impact of Timing on Discharge to Death or Discharge to Hospice
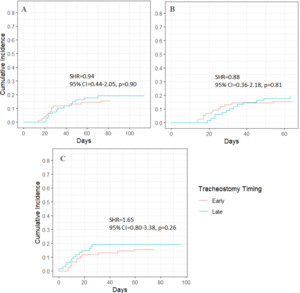
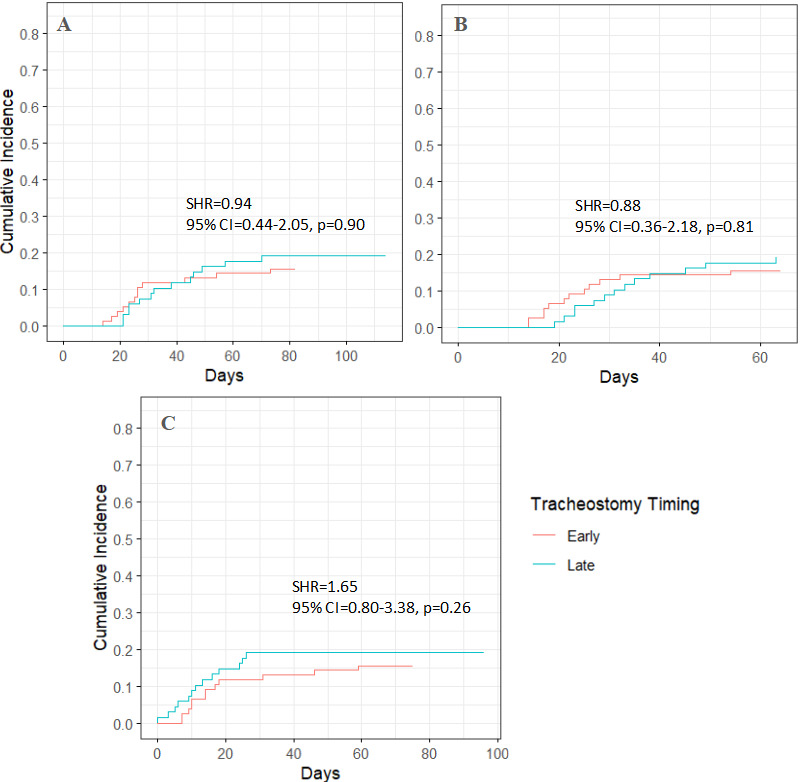
In stroke patients, late tracheostomy was not significantly associated with death or discharge to hospice (p = 0.90, 0.81, and 0.26 across different models) (Figure 4; Table S5). In non-stroke patients, late tracheostomy was also not significantly associated with death or discharge to hospice (p = 0.11, 0.08, and 0.79), though a trend toward lower risk was observed (Figure 5; Table S6).
DISCUSSION
The findings of this study demonstrate that early tracheostomy (<14 days) in neurocritical patients, particularly those with stroke, is associated with significantly shorter ICU and hospital stays, lower reintubation rates, and reduced healthcare costs compared to late tracheostomy (>14 days). Stroke patients who underwent early tracheostomy were 40% more likely to be discharged to rehabilitation, while those who received late tracheostomy had a 2.7-fold increased risk of reintubation, highlighting the potential benefits of earlier airway management in this population. Among non-stroke patients, late tracheostomy was associated with prolonged ICU stays (35.5 vs. 22.1 days, p < 0.01), extended hospitalization (50.8 vs. 32 days, p < 0.01), and significantly higher hospital costs ($206,184 vs. $128,788, p < 0.01). While early tracheostomy was linked to a higher rate of procedure-related complications in stroke patients (22.1% vs. 7.3%, p = 0.03), this risk was most likely outweighed by the benefits of earlier ventilator liberation and discharge. These findings highlight the importance of optimizing tracheostomy timing to improve clinical outcomes, streamline ICU resource utilization, and facilitate earlier rehabilitation in neurocritical patients.
Findings from this retrospective study align with other literature demonstrating that stroke patients admitted to the ICU have severe neurological impairment, leading to a higher risk of mortality than non-stroke critical care patients.14,27 The non-stroke cohort also included patients with a variety of neurological conditions, many of which carry less known long-term disability as compared with the cohort with severe stroke. Findings from this retrospective study also contrast with those presented in a 2023 meta-analysis and two randomized trials in critically ill stroke patients that yielded no association of tracheostomy timing with ICU LOS.14,16 This discrepancy may be related to differences in definitions of tracheostomy timing: these studies defined early and late tracheostomy as <5 and >10 days, respectively, whereas we demarcated at 14 days. The decision to delay tracheostomy beyond 14 days may reflect greater patient complexity. The 27.6-day average ICU LOS among late tracheostomy patients in our study is notably longer than the 16.4-day average ICU LOS reported in the meta-analysis.14 The association of tracheostomy timing with ICU and hospital LOS may involve other factors including complication rates, surgical intervention, and eligibility for fibrinolysis that were not included in this analysis. Regardless, these findings suggest potential benefit for tracheostomy before the 14-day time point.
Implications for rehabilitation (Acute/Chronic)
Early placement of a tracheostomy plays a significant role in facilitating quicker liberation from mechanical ventilation and expediting discharge from the inpatient setting to rehabilitation.28–31 This approach aims to provide stroke patients with timely access to rehabilitation during critical recovery windows. In addition, expedited liberation from the ventilator can reduce the risk of delirium and post-intensive care syndrome, which involves survivorship considerations of physical, cognitive, and mental health recovery.32 Despite the potential benefit of accelerated liberation from the ventilator, the presence of a tracheostomy can become a barrier to admission at rehabilitation facilities, particularly if these facilities are unable to manage patients with a tracheostomy. This creates a challenging dilemma for clinicians, who must balance the priorities of ensuring patient safety, accelerating discharge, and optimizing recovery from the debilitating effects of stroke.
Stroke rehabilitation centers range in services provided but are designed to deliver tailored neurorehabilitation programs, including intensive therapy sessions, incorporating advanced technologies like robotic-assisted therapy and functional electrical stimulation to optimize motor recovery. The structured, interdisciplinary resources available at such facilities ensure that patients receive comprehensive care designed to maximize their recovery potential. A majority of stroke patients attend general rehabilitation centers that focus on therapy to expedite recovery and identify safe, adaptive solutions to support independence. These centers provide physical therapy to promote safe mobility, occupational therapy to address recovery toward functional independence, and speech therapy to assist with cognitive, language and swallowing issues common after stroke. Subacute rehabilitation centers and skilled nursing facilities provide more supportive care with less time spent on specific recovery therapies like the ones delivered at acute inpatient rehabilitation centers. These centers provide daily care focused on the prevention of complications such as contractures and support with nutrition with some therapy focused on safety and mobility.33
The addition of a tracheostomy adds complexity to rehabilitative care and reduces the number of centers equipped to provide this combination of specialized services. Addressing tracheostomy care in the hospital setting and throughout the rehabilitation process requires interprofessional collaboration.34,35 Clinicians across disciplines, including physicians, nurses, respiratory therapists, speech-language pathologists, and case managers have a role in care. The Global Tracheostomy Collaborative (GTC) supports standardizing best practices, enhancing interprofessional training, fostering data-driven improvements, and actively engaging patients and care partners.36–39 This approach has achieved significant improvements in patient outcomes and cost savings. For the stroke population, practical steps include team-based approaches ensuring that tracheostomy care plans prioritize decannulation when feasible and equipping rehabilitation facilities with the knowledge and resources to manage patients with a tracheostomy effectively. By bridging gaps in care and advocating for patient-centered solutions, interprofessional efforts can help overcome barriers to recovery, maximizing timely access to needed rehabilitation.30
Preventing, Recognizing, and Managing Complications
Despite expedited hospital courses and reduced total costs, early tracheostomy was associated with higher rates of tracheostomy-related complications. This contrasts with existing evidence demonstrating unchanged or decreased complications with early tracheostomy.15,40–42 Previous studies have focused on VAP and sepsis as complications; however, the present study incorporated several other complications not represented in most studies, such as tracheitis, stomal granulation, cutaneous infection, and non-aspiration pneumonia. Such complications in the early recovery period after stroke are critical to understand since this is the period of most efficient brain recovery. In the early recovery period after stroke, infections or complications are likely to have a long-term impact on functional outcomes.43 This finding of higher rates of complication with early tracheostomy is especially notable since we suspect that the actual number of complications is even higher considering the limitations common to our retrospective clinical data set and poor documentations.43
Complications in patients with tracheostomy span a spectrum from acute, life-threatening events to more chronic, insidious injuries that develop over time. Acute complications, such as tube obstruction, occlusion, dislodgement, and acute bleeding, represent critical emergencies that demand immediate intervention. These events can result in rapid respiratory compromise, highlighting the need for vigilant monitoring and prompt response protocols. In contrast, more chronic complications such as pressure injuries, granulation tissue formation, tracheal erosions, and infections often emerge over weeks to months of tracheostomy use.44,45 These chronic injuries can significantly impact long-term outcomes, requiring interdisciplinary management and sometimes surgical interventions. Implementing broad-based education and standardized protocols may reduce risk.46,47 The variable complication types highlight the role of both acute and chronic care strategies, tailored to address the evolving needs of patients with a tracheostomy across their care trajectory.
Although not assessed in this study, early versus late tracheostomy can also have significant survivorship implications due to the sequelae of prolonged translaryngeal intubation and sedation.48–51 Laryngotracheal injury can predispose to dysphagia, altered speech, and stenosis that significantly affects breathing and overall quality of life for tracheostomy patients. Dysphagia, often associated with impaired laryngeal elevation and altered dynamics, increases the risk of aspiration and impacts nutritional intake. Altered speech, due to the inability to generate phonation through the vocal cords, poses additional communication challenges, even with interventions such as speaking valves.52 Moreover, laryngotracheal stenosis—a chronic sequela of tracheostomy—can lead to substantial morbidity, often requiring complex surgical reconstruction. These issues, combined with psychosocial impacts such as isolation and emotional distress, highlight the importance of holistic care models. Addressing these survivorship concerns necessitates a collaborative, interdisciplinary approach involving speech-language pathologists, pulmonologists, otolaryngologists, and other specialists to optimize long-term outcomes and enhance the overall well-being of patients recovering from critical illnesses.
Limitations
Our analysis has several limitations. First, data were derived from a single quaternary center and included patients who had both PEGs and tracheostomies. This may introduce selection bias due to increased complexity relative to the tracheostomy patient population. Second, the data set is derived from the clinical record specific to the patient’s hospital course, clinical characteristics, and decision-making process surrounding tracheostomy intervention, which limits our ability to understand the factors influencing clinical decision making and outcomes. The analysis did not control for socioeconomic status, stroke subtype, tracheostomy indication, and individual comorbidities. Third, the retrospective nature of this study necessarily excludes patients who may have been candidates for tracheostomy but did not ultimately receive one. Fourth, other studies define time to tracheostomy as the duration between initiation of mechanical ventilation and tracheostomy, but due to data availability we defined our start point as time to admission, biasing towards longer lead time to tracheostomy. Last, the use of discharge disposition as an outcome reflects significant heterogeneity in rehabilitation facilities and does not provide insight long-term neurological, mortality, or quality of life outcomes beyond hospital course.
Generalizability
Our study benefits from a large, consecutive dataset and a robust analytical approach employing competing risks analysis, which offers more detailed and accurate insight than traditional regression methods. The cohorts were well-balanced demographically, were managed by the same group of clinical neuroscience ICU providers, and the inclusion of multiple parameters offers relatively comprehensive insight into patient characteristics and outcomes. However, the variety of stroke and non-stroke neurological diagnoses included in our study may limit the generalizability of our findings to specific patient subgroups. Future research focusing on specific stroke subtypes or non-stroke diagnoses may provide more tailored insights. Despite these limitations, our study provides valuable insight into the relationship between stroke status, tracheostomy timing, and patient outcomes. Our findings emphasize the need for individualized decision-making regarding tracheostomy timing, carefully weighing the potential benefits of earlier intervention against the risk of complications, echoing the ongoing debate in the field. Future prospective studies are needed to further refine our understanding of optimal tracheostomy management in this complex patient population.
Future Directions
Future research should include all patients with severe stroke phenotype to understand the natural history toward tracheostomy placement and indications. A prospective, multicenter study in a more homogeneous stroke population is important to validate the findings from this retrospective analysis and to address unresolved questions regarding tracheostomy timing in neurocritical care populations. Trials that incorporate standardized definitions for early and late tracheostomy and stratify stroke patients by subtype (e.g., ischemic, hemorrhagic, or subarachnoid) could provide more granular insights into optimal timing and associated outcomes. Additionally, studies should include long-term measures of post-hospital recovery treatments, patient complications, and quality-of-life outcomes, including functional recovery, speech, swallowing, and psychosocial well-being, to better understand the broader implications of tracheostomy timing. Incorporating patient-centered metrics, such as health-related quality of life and caregiver burden, will ensure that future recommendations align with the needs and priorities of patients and their families.
Another critical avenue for exploration is the role of interprofessional care models, such as those championed by the GTC, in improving outcomes for stroke and other neurocritical care patients. Future initiatives should assess the impact of standardized protocols, enhanced training for rehabilitation staff, and expanded access to decannulation resources at rehabilitation facilities. Leveraging real-world data from global registries could identify best practices for managing tracheostomy-related complications and ensuring timely transitions to appropriate levels of care.49 In parallel, the integration of emerging technologies—such as artificial intelligence for risk prediction and virtual reality for caregiver training—offers opportunities to enhance the safety and effectiveness of tracheostomy management across diverse care settings. These efforts will contribute to a more personalized and equitable approach to tracheostomy care, advancing recovery outcomes for neurocritical care patients.
Conclusion
This study highlights the importance of timely tracheostomy placement in neurocritical care patients, particularly among those with stroke. Our findings suggest that early tracheostomy may improve ICU efficiency, reduce reintubation risk, and facilitate earlier rehabilitation, ultimately contributing to better recovery trajectories. Future research should focus on developing predictive models for tracheostomy timing and assessing long-term functional outcomes in stroke and non-stroke neurocritical patients. Ultimately, a personalized, evidence-based approach to tracheostomy timing could optimize patient outcomes while reducing healthcare burden in critically-ill populations.
Funding
1. NIH 5R01NR017433 and AHRQ 5R18HS029124 (Vinciya Pandian)
2. University of Michigan Medical School Research Grant
Conflicts of Interest
None
Presentation
Preliminary data were presented at the 48th Society of Otorhinolaryngology and Head-Neck Nurses’ Annual Congress and Educational Symposium on October 19th in Baltimore, MD; and at the 8th International Tracheostomy Symposium, Inspiring Advances in Global Tracheostomy Care on November 1st, virtually.