INTRODUCTION
Tracheostomies are critical medical procedures performed to secure an airway, particularly for patients with severe respiratory or neurological conditions.1,2 While these interventions can be life-saving, they are associated with complex care needs and significant long-term health implications.3–5 Proper maintenance and follow-up care are essential to prevent complications, optimize clinical outcomes, and enhance patients’ quality of life (QoL).6–9 However, gaps in understanding the impact of follow-up care on tracheostomy management remain, underscoring the need for evidence-based, multi-disciplinary, and standardized practices to guide post-discharge care.
Despite advancements in surgical techniques and technology, individuals with a tracheostomy continue to face numerous challenges when discharged with a tracheostomy in situ, including stoma infections,10–12 speech13–16 and swallowing difficulties,17,18 and respiratory complications.19–21 Adherence to recommended tracheostomy care routines, such as regular stoma cleaning and tube changes, is critical to mitigating these risks.22–25 Furthermore, the presence or absence of structured follow-up care may significantly influence patients’ long-term outcomes, including tracheostomy tube retention, stoma closure, and health-related quality of life (HRQoL).26–29 Limited data exist on the role of follow-up care in reinforcing adherence to care routines, reducing complications, and improving QoL outcomes for tracheostomy patients.
This study aims to address this gap by evaluating the impact of follow-up care on tracheostomy maintenance practices, clinical outcomes, and QoL at both three months and three years post-discharge. Specifically, this investigation compares patients who received follow-up care with those who did not, examining differences in adherence to tracheostomy care routines, complication rates, and HRQoL measures. By identifying key factors influencing these outcomes, the findings will provide valuable insights to inform best practices and improve the standard of care for tracheostomy patients.
Objectives
The primary objective of this study is to evaluate the role of follow-up care in enhancing adherence to tracheostomy maintenance practices, reducing clinical complications, and improving long-term QoL outcomes. This study hypothesizes that patients who receive follow-up care will demonstrate better adherence, fewer complications, and higher QoL scores than those who do not.
Specific aims include:
-
Assessing adherence to tracheostomy maintenance practices, including the frequency of stoma cleaning and tube changes, as well as self-reported adherence to care routines.
-
Investigating tracheostomy-related outcomes and challenges, such as stoma infections, respiratory issues, and the need for additional interventions.
-
Evaluating long-term outcomes, including tracheostomy tube retention, stoma closure, cosmetic results, and QoL measures at three months post-discharge.
By addressing these aims, this study seeks to provide a comprehensive understanding of how follow-up care influences the trajectory of tracheostomy recovery and long-term health.
METHODS
Study Design and Setting
This prospective longitudinal cohort study evaluated the impact of follow-up care on tracheostomy maintenance practices, clinical outcomes, and quality of life three months post-discharge. The study was conducted at The Johns Hopkins Hospital (JHH), an academic medical center with over 1,100 beds, including a dedicated otolaryngology service and intensive care units. Tracheostomy care at discharge included standardized education on stoma care, suctioning techniques, and tube change frequency, provided by interprofessional team (Supplemental Figure 1). Across the 10-year period, tracheostomy care practices evolved, including the formalization of a multidisciplinary tracheostomy team in 2007.30,31 The study included all patients who underwent an open or percutaneous tracheostomy between July 2007 and June 2017. Data collection included a review of electronic health records and administration of telephone surveys and continued until 2020.
Participants
Adult patients who underwent a tracheostomy at The Johns Hopkins Hospital and were alive at the time of the survey were included in the study. Non-English-speaking patients and those with hearing impairments that precluded effective telephone communication were excluded. Patients were identified through institutional records, and survival status at three months post-discharge was confirmed using the Social Security Death Index.32 Eligible participants were contacted via telephone to obtain informed consent. For those unable to participate by phone, surveys and consent forms were mailed. Up to three follow-up attempts were made to maximize participation. Participants were contacted at 3 months and 3 years to gather additional information. This study included all adult patients who underwent tracheostomy at the JHH between July 2007 and June 2017 and were alive at the time of follow-up. JHH performs about 150 – 200 tracheostomies per year, and this sample represents a subset of those who could be contacted and consented.
Variables
Key variables in the study included outcomes such as adherence to tracheostomy care (measured by the frequency of stoma cleaning and tube changes), clinical complications (e.g., stoma infections, swallowing difficulties, and breathing issues), and HRQoL assessed using SF-8™ Health Survey scores.33 The primary exposure was the receipt of follow-up care. Follow-up care was defined as any post-discharge clinical evaluation by a healthcare provider specifically addressing tracheostomy management within the first three months after hospital discharge. This included visits with tracheostomy nurse practitioners (NPs), otolaryngologists, pulmonologists, intensivists, or other proceduralists involved in tracheostomy management. Home health nursing visits were not considered follow-up care unless they were part of a structured tracheostomy clinic visit led by a qualified provider specializing in tracheostomy management. General medical follow-ups that did not address tracheostomy-specific concerns were also excluded from this category. Predictors and potential confounders included demographic factors (age, sex, race), clinical variables (underlying diagnoses and type of tracheostomy), and the timing and frequency of follow-up care.
Data Sources and Measurements
Data sources included electronic health records and structured telephone surveys. Surveys assessed adherence to stoma cleaning and tracheostomy tube changes, complications (Supplemental Figure 2), and HRQoL using the SF-8 instrument.33 The authors secured the non-commercial license agreement (number QM047404) for the SF-36V2 from OptumInsight Life Sciences, Inc. and utilized the QualityMetric Health Outcomes Scoring Software 5.0. Consistency in survey administration was maintained across participants to ensure comparability. The SF-8 Health Survey is a reliable and valid tool for assessing HRQoL across eight domains, with strong internal consistency (Cronbach’s alpha >0.80), test-retest reliability, and responsiveness to health changes.34,35 Its norm-based scoring (mean 50, SD 10) and concise Physical and Mental Component Summaries make it practical for clinical and research use, requiring only 2-3 minutes to complete.
Bias Mitigation
To address potential selection bias, all eligible participants meeting inclusion criteria were systematically approached, and multiple contact attempts were made. Response bias was minimized through standardized survey instruments. Missing data were managed by excluding incomplete responses from specific analyses, with sensitivity analyses conducted where applicable.
Study Size
The study included all eligible patients identified between 2007 and 2017. A total of 2,070 patients were screened, with 1,408 eligible based on survival status. Of these, 220 completed the survey and formed the final cohort for analysis. The sample size was determined by the availability of eligible patients and anticipated response rates.
Quantitative Variables
Continuous variables such as HRQoL scores were analyzed using their means and standard deviations. Categorical variables, including frequency of stoma cleaning and tube changes, were presented as percentages. Groupings for categorical variables were predefined based on clinical relevance (e.g., daily, weekly).
Statistical Methods
Statistical methods included descriptive analyses to calculate means, standard deviations, frequencies, and percentages. Comparative analyses were conducted between follow-up and no follow-up groups using chi-square tests for categorical variables (with Fisher’s exact test for small cell sizes) and independent t-tests for continuous variables. Multivariate analysis, employing multiple linear regression with backward elimination, was used to identify predictors of HRQoL outcomes, with covariates including age, sex, race, follow-up care status, stoma infection, speech, swallowing, hoarseness, and difficulty with breathing. Surveys with missing critical responses were excluded from specific analyses, and sensitivity analyses were performed to evaluate the impact of missing data.
Ethical Considerations
The Johns Hopkins Institutional Review Board approved the study (IRB00133797). Informed consent was obtained either verbally (phone surveys) or in writing (mailed surveys). Participant confidentiality was maintained through secure data storage and anonymization.
RESULTS
Eligibility and Participation Rates in Tracheostomy Follow-Up Study
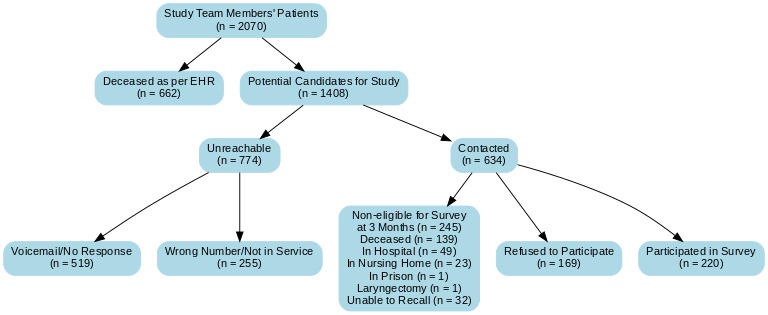
A total of 2,070 patients were identified from the study team’s records (Figure 1). Of these, 662 (32%) were confirmed deceased as per the health records, leaving 1,408 potential candidates for the study. Among these, 774 individuals (55%) were unreachable, with the primary reasons being lack of response to voicemail (519, 67%) and incorrect or inactive phone numbers (255, 33%). The remaining 634 candidates were contacted, and 245 (39%) were deemed ineligible for the survey at three months. Reasons for ineligibility included being deceased (139, 57%) (verified by next of kin), currently hospitalized (49, 20%), residing in a nursing home (23, 9%), incarcerated (1, <1%), underwent laryngectomy (1, <1%), or being unable to recall (32, 13%). Of the eligible participants, 169 (27%) refused to participate, and 220 (35%) completed the survey, representing the final cohort for data analysis. Even though consent forms and surveys were mailed, all of them responded to the surveys by phone.
Demographic and Clinical Characteristics of Study Participants
The 220 participants had a mean age of 56.0 ± 17.6 years, comprising 123 men (55.9%) and 97 women (44.1%). Most patients were Caucasian (130, 59.1%), followed by African American (74, 33.6%), Middle Eastern (12, 5.5%), and Hispanic and Asian (2 each, 0.9%). Tracheostomy procedures were performed percutaneously in 86 patients (39.1%) and via open surgery in 134 patients (60.9%). Patients who received follow-up (n = 166) had a mean age of 55.0 ± 18.1 years compared to 58.8 ± 15.9 years in those without follow-up (n = 54, p = 0.17). Among follow-up patients, 57.2% (95) were men, and 42.8% (71) were women, while the no follow-up group included 51.9% (28) men and 48.2% (26) women (p = 0.49). Racial composition was comparable between groups, with Caucasians comprising 58.4% (97) of the follow-up group and 61.1% (33) of the no follow-up group, African Americans 34.3% (57) and 31.5% (17), and Middle Eastern patients 4.8% (8) and 7.4% (4), respectively. Hispanic and Asian patients (1.2%, 2 each) were present only in the follow-up group. These differences were not statistically significant (p = 0.75).
Follow-Up Care and Its Influence on Tracheostomy Maintenance Adherence
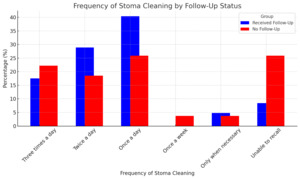
The frequency of stoma cleaning differed significantly between the two groups (p = 0.001). Among participants with follow-up care, the most common frequencies of stoma cleaning were once a day (67, 40.4%), twice a day (48, 28.9%), and three times a day (29, 17.5%) (Figure 2). In contrast, participants without follow-up care most commonly cleaned the stoma once a day (14, 25.9%) or three times a day (12, 22.2%). A significantly higher percentage of participants without follow-up care were unable to recall their stoma cleaning frequency (14, 25.9%) compared to those with follow-up care (14, 8.4%).
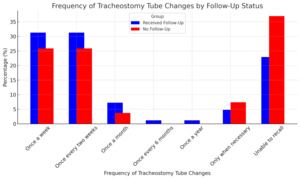
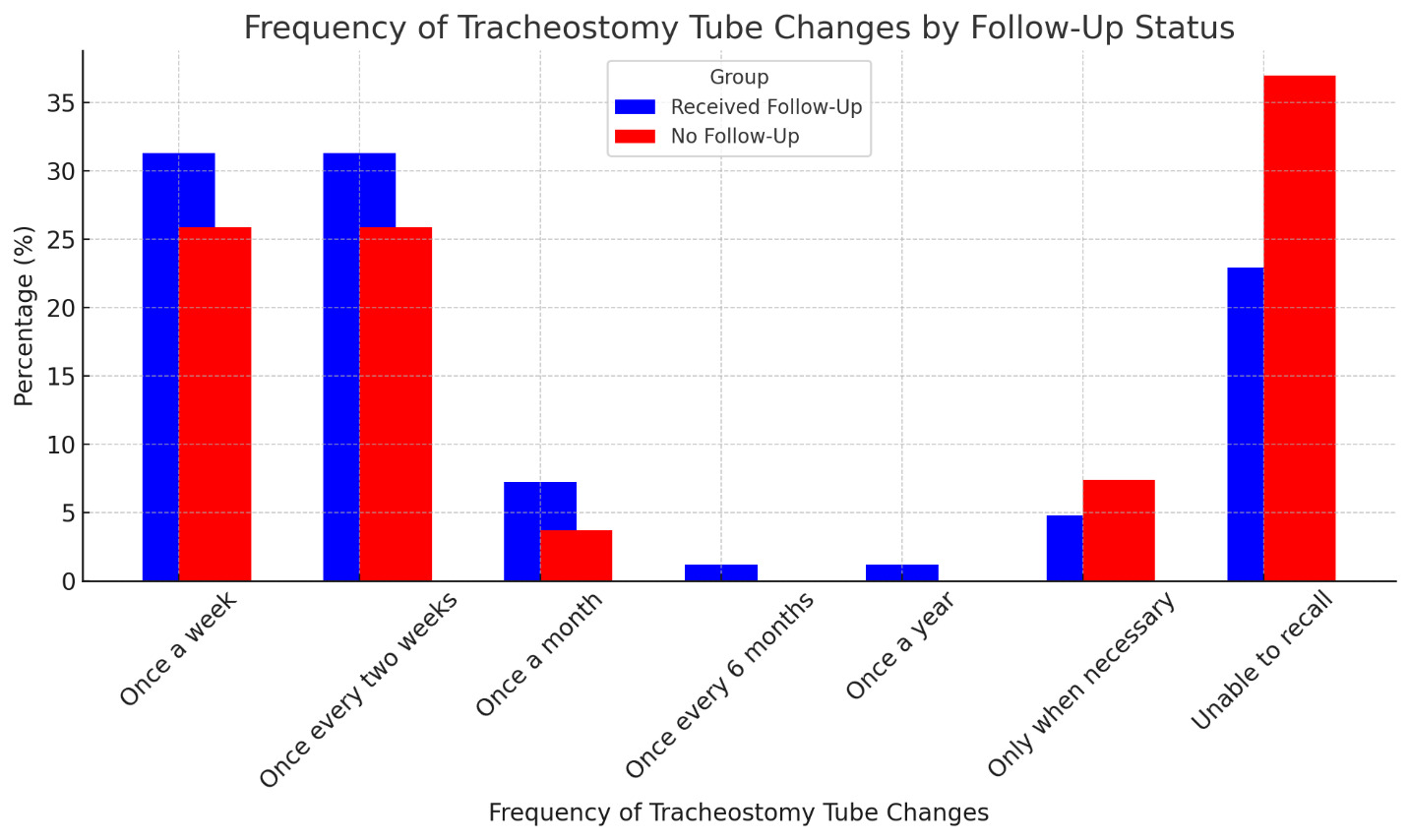
The frequency of tracheostomy tube changes did not differ significantly between the two groups (p = 0.37) (Figure 3). In both groups, the most common frequencies were once a week (52, 31.3% in follow-up; 14, 25.9% in no follow-up) and once every two weeks (52, 31.3% in follow-up; 14, 25.9% in no follow-up). However, participants without follow-up care were more likely to be unable to recall their tracheostomy tube change frequency (20, 37.0%) compared to those with follow-up care (38, 22.9%).
Clinical Outcomes and Breathing Challenges at 3 Months Post-Discharge
Among the 220 participants, the incidence of stoma infections was 6.4% (14 cases). Speech-related issues were reported by 20.9% (24) of participants as occasional and 9.6% (21) as persistent, with the remaining 79.6% (175) reporting no issues. Swallowing difficulties were reported by 10.5% (23) as occasional and 13.2% (29) as persistent, while 76.4% (168) had no swallowing issues. The use of a feeding tube was noted in 40.9% (90) of participants. Participants with follow-up care had a higher incidence of stoma infections (7.8% vs. 1.9%, p = 0.11) and greater use of feeding tubes (43.4% vs. 33.3%, p = 0.19), though these differences were not significant (Table 1). Speech issues were more common in the follow-up group, with occasional (13.3% vs. 3.7%) and persistent (10.2% vs. 7.4%) problems, but most participants in both groups reported no issues (76.5% vs. 88.9%, p = 0.10). Swallowing difficulties were more frequent in the follow-up group (26.6% vs. 14.8%, p = 0.20).
Hoarseness (voice issues) was reported as occasional by 21.8% (48) and persistent by 13.2% (29), with 65.0% (143) reporting no hoarseness. Difficulty breathing was noted occasionally by 27.3% (60) and persistently by 7.7% (17), while 65.0% (143) had no breathing difficulties. Hoarseness was reported occasionally (24.7% vs. 13.0%) or persistently (13.9% vs. 11.1%) in follow-up participants, while no hoarseness was reported by 61.5% and 75.9%, respectively (p = 0.13). Breathing difficulties were significantly higher in the follow-up group (39.1% vs. 22.2%, p = 0.05) (Table 1).
Factors Contributing to Breathing Challenges in Tracheostomy Patients
Among the 77 participants who reported difficulty breathing, the primary causes were pulmonary conditions (44.2%, 34), airway or tracheostomy tube-related issues (22.1%, 17), activity or exercise-induced factors (14.3%, 11), cardiovascular conditions (13.0%, 10), and neurological causes (6.5%, 5). Pulmonary causes were more common in the follow-up group (49.2% vs. 16.7%), while airway or tracheostomy tube-related issues were more frequent in the no follow-up group (50.0% vs. 17.9%, p = 0.07) (Table 1).
Half of these participants (50.7%, 39) were receiving breathing treatments, and 23.4% (18) required mechanical ventilation. Most participants with breathing difficulties did not require invasive procedures (83.1%, 64) (Table 1). However, 11.7% (9) underwent surgical interventions, and 5.2% (4) required airway stenting. More participants with follow-up required mechanical ventilation (27.7% vs. 0%, p = 0.04). Most participants did not require additional procedures (80.0% vs. 100%, p = 0.24), though surgical interventions (13.9%) and airway stenting (6.2%) occurred only in the follow-up group.
Impact of Follow-Up Care on Quality of Life at 3 Months Post-Discharge
Physical functioning had a mean score of 57 ± 40.0, indicating moderate limitations in physical activities. Role physical, reflecting difficulties with daily activities due to physical health, was relatively low at 27.4 ± 26.2. Bodily pain scored higher at 73.1 ± 31.8, suggesting less interference from pain in daily life. General health was rated at 65.4 ± 28.2, reflecting a generally positive perception of health. The no-follow-up group reported higher scores in physical functioning (65.7 vs. 54.5, p = 0.06), physical role (32.9 vs. 25.6, p = 0.07), and bodily pain (82.4 vs. 70.0, p = 0.01) (Table 2).
Vitality, measuring energy levels, scored 54.5 ± 25.3, indicating moderate levels of fatigue. Social functioning, which evaluates the impact of health on social interactions, scored 66.5 ± 28.2, while role emotional, assessing the influence of emotional health on daily roles, was lower at 36.2 ± 31.9. Mental health, representing emotional well-being, had a mean score of 61.9 ± 24.7. General health (64.2 ± 27.6 vs. 69.1 ± 29.9, p = 0.27) and vitality (53.2 ± 24.8 vs. 58.8 ± 26.7, p = 0.16) were comparable between the groups. Social functioning scores were similar (67.2 ± 27.5 vs. 65.7 ± 30.4, p = 0.74), as were mental health scores (61.4 ± 24.2 vs. 63.3 ± 26.4, p = 0.61).
Composite scores revealed a mean Physical Component Summary (PCS) of 45.3 ± 10.3, reflecting moderate physical health, and a Mental Component Summary (MCS) of 42.2 ± 11.0, indicating somewhat lower mental health compared to physical health. Composite scores showed a significantly higher PCS in the no-follow-up group (48.0 vs. 44.4, p = 0.03), while the MCS was comparable between groups (42.8 vs. 42.1, p = 0.66).
A multiple linear regression analysis identified predictors of PCS scores at 3 months post-discharge. The final model retained five significant predictors—age, sex, follow-up care, hoarseness, and swallowing issues—explaining 32.0% of the variance (R-squared = 0.32, p < 0.001). PCS scores decreased by 0.11 points per year of age (p = 0.002) and were 3.2 points lower in males than females (p = 0.008). Follow-up care was associated with a 2.8-point increase in PCS scores (p = 0.044), while hoarseness and swallowing issues reduced scores by 1.9 (p = 0.03) and 5.3 points (p < 0.001), respectively.
For MCS scores, the final model included hoarseness, wound infections, difficulty breathing, and speech issues, explaining 8% of the variance (R-squared = 0.08, p = 0.001). Hoarseness was unexpectedly associated with a 5-point increase (p < 0.001), while wound infections and speech issues reduced scores by 6.3 (p = 0.044) and 3.5 points (p = 0.02), respectively. Difficulty breathing showed a modest 1.8-point increase in MCS scores (p = 0.03).
Patterns and Timing of Follow-Up Care Post-Tracheostomy
Of the 220 participants, 75.5% (166) attended at least one follow-up visit, while 24.6% (54) did not. Among those who attended follow-up, the timing of the first post-discharge visit varied: 18.1% (30) were seen within one week, 13.3% (22) within two weeks, 40.4% (67) within one month, and 15.7% (26) within six months. A smaller proportion had their first follow-up within one year (6.6%, 11) or after more than a year (6.0%, 10). Endoscopic evaluations were performed during the first visit for 19.3% (32) of participants. The type of healthcare professional seen at the first follow-up was most commonly an otolaryngologist (54.8%, 91), followed by a general surgeon (23.5%, 39), pulmonologist (10.2%, 17), or internal medicine physician (6.0%, 10). A small percentage (5.4%, 9) could not recall the provider. The frequency of follow-up visits varied, with 31.3% (52) attending every three months, 23.5% (39) monthly, and 10.8% (18) every six months. Annual follow-ups were reported by 11.5% (19), while 13.9% (23) reported attending only a single visit. Less frequent follow-ups included weekly (4.2%, 7) or every two weeks (4.8%, 8). The timing of the last follow-up visit also showed variability: 10.2% (17) occurred within one month of discharge, 3.6% (6) within six months, 24.7% (41) within one year, 44.6% (74) within two years, and 16.9% (28) more than two years post-discharge.
Long-Term Tracheostomy Tube Retention
Three years post-discharge, 6.8% (15/220) of participants still had their tracheostomy tubes in place, while the remaining 93.2% (205/220) had their tracheostomy tubes removed (Figure 4). Among those who retained their tracheostomy tubes (n = 15), the most common reason was tracheal stenosis, accounting for 33.3% (5/15) of cases. Other reasons included respiratory distress (13.3%, 2), difficulty breathing (13.3%, 2), obstructive sleep apnea (13.3%, 2), neuromuscular weakness (6.7%, 1), and musculoskeletal deformity (6.7%, 1). In 13.3% (2) of cases, the reason for retention was unknown.
Long-Term Tracheostomy Outcomes: Tube Removal, Stoma Closure, and Cosmetic Results
Among 205 participants who underwent tracheostomy tube removal, the most common timing was within 1–6 months post-discharge (34.6%), followed by 7–12 months (27.3%), before discharge (17.6%), within one month (10.7%), and more than a year after discharge (8.8%). Only 1.0% were unable to recall. Timing of removal differed significantly between groups (Table 3, p < 0.001). Participants with follow-up care (n = 151) were most likely to have their tubes removed 1–6 months (33.1%) or 7–12 months (30.5%) post-discharge. In contrast, those without follow-up (n = 54) more often had removals before discharge (35.2%) or within 1–6 months (38.9%). Removals after more than one year occurred exclusively in the follow-up group (11.9%).
The majority of tracheostomy tube removals occurred in hospitals (47.8%), followed by acute (18.1%) and sub-acute rehabilitation facilities (18.1%), skilled nursing facilities (10.2%), and long-term ventilator facilities, or at home (2.9% each). Hospital settings were the most common location for removal in both groups (48.3% with follow-up vs. 46.3% without, p = 0.005). Acute rehabilitation facilities accounted for 21.9% of removals in the follow-up group, while sub-acute facilities were more frequent in the no-follow-up group (29.6%).
Stoma closure most frequently occurred within one week (25.4%), followed by three days (21.5%), two days (18.1%), and one month (10.7%). Smaller proportions reported closure within two weeks (8.8%), three months (5.4%), three weeks (3.3%), or within one day (3.9%). Persistent open stomas were reported in 2.0% of participants, exclusively in the follow-up group. Timing of stoma closure differed significantly between groups (p = 0.001). Closure within one week was common in both groups (26.5% with follow-up; 22.2% without), while closure within one day was more frequent in the no-follow-up group (11.1% vs. 1.3%).
Cosmetic outcomes of the stoma site varied, with 40.0% of participants reporting a ½ inch scar, 28.3% a ¼ inch scar, 13.7% a ¾ inch scar, 8.9% a scar larger than one inch, and 9.3% no visible scar. Plastic surgery assistance for stoma closure was utilized by 4.9% of participants. Follow-up care for stoma healing was reported by 38.1% of participants overall and was slightly more common in the follow-up group (41.1% vs. 29.6%, p = 0.20).
DISCUSSION
This study evaluated the impact of follow-up care on tracheostomy management, clinical outcomes, and quality of life three months post-discharge. Follow-up care significantly improved adherence to tracheostomy maintenance practices, particularly in stoma cleaning, although it did not affect tube change frequency. While participants receiving follow-up care reported higher incidences of clinical complications such as stoma infections, breathing difficulties, and persistent voice or swallowing issues, these differences were not statistically significant except for breathing difficulties. QoL measures showed marginally better physical health in the no-follow-up group, with comparable mental health scores across groups. Long-term outcomes evaluated at three months and three years post-discharge, including tracheostomy tube retention and stoma closure timing, were significantly influenced by follow-up care, as those receiving follow-up were more likely to undergo timely tube removal and report favorable cosmetic results.
Strengthening Tracheostomy Care: The Role of Follow-Up in Enhancing Adherence
This study highlights the importance of follow-up care in improving adherence to tracheostomy maintenance, particularly stoma cleaning, with participants demonstrating significantly higher adherence rates and fewer lapses in care.36,37 Patients who sought follow-up care may have been more informed or motivated to adhere to best practices for tracheostomy management, whereas those who did not follow up may have had barriers such as logistical challenges, lack of awareness, or lower health literacy. However, the lack of improvement in tube change frequency indicates gaps in caregiver education and communication requiring targeted interventions.8,36,38,39 Structured follow-up ensures timely monitoring and reduces complications, and telehealth may serve as a scalable solution for underserved areas.40,41 Effective discharge planning and holistic approaches addressing both biological and psychosocial needs further enhance outcomes, particularly for patients with comorbidities.42–44 Structured follow-up care significantly improves adherence and outcomes, warranting integration of multidisciplinary teams, telehealth, and tailored discharge protocols. Further studies should evaluate how patient characteristics, health literacy, and social determinants of health influence follow-up engagement and whether targeted interventions, such as telehealth or patient education programs, could mitigate disparities in access and adherence.
Navigating Complications: Breathing Challenges and Clinical Outcomes Post-Tracheostomy
Breathing difficulties were significantly more common in the follow-up group compared to the no-follow-up group, with pulmonary conditions being the primary cause among follow-up participants. The higher incidence in the follow-up group likely reflects more frequent identification of complications due to closer monitoring, as regular follow-up allows for earlier detection and management of respiratory issues.45 Variations in tracheostomy tube sizing and fit have been shown to increase the work of breathing, further emphasizing the importance of follow-up to identify issues with tube and positioning.46 Patients requiring mechanical ventilation in our study were particularly likely to adhere to follow-up care, underscoring the critical role of structured programs in managing severe complications.47 These programs ensure that complications such as pulmonary and airway issues are addressed promptly, reducing the risk of rehospitalization and improving outcomes.48 Additionally, follow-up care provides opportunities for tailored interventions, such as cuff deflation strategies, to alleviate respiratory effort and enhance recovery.49
Follow-Up Care and Quality of Life: Assessing Physical and Mental Health Impacts
This study highlights the complex relationship between follow-up care and QoL, with marginally better physical health scores observed in the no-follow-up group and comparable mental health outcomes across groups, possibly reflecting baseline differences or variability in follow-up care quality.47 The higher self-reported QOL scores in the no-follow-up group may reflect differences in independence, baseline health status, or perception bias. Patients managing their tracheostomy independently may be inherently healthier or more motivated to perceive themselves as healthier. Structured, multidisciplinary follow-up programs have been shown to improve QoL by addressing post-ICU challenges such as pain, mobility, and emotional well-being.49,50 Vargas et al. (2024) highlighted that tracheostomized ICU survivors often experience significantly compromised QoL up to 12 months post-discharge, emphasizing the importance of tailored interventions during early follow-up.29 Predictors of PCS scores, including swallowing difficulties and voice issues (hoarseness), align with findings from McCormick et al. (2015), who stressed the critical role of patient and family education in managing such challenges.51 Notably, hoarseness was unexpectedly associated with improved MCS scores in this study, a finding that may reflect adaptive resilience.52 These results show the need for comprehensive, patient-centered follow-up systems to optimize long-term recovery.47,53
Addressing Voice and Speech Challenges in Tracheostomy Follow-Up Care
Difficulties with voice and speech were common among study participants, highlighting the lasting impact of tracheostomy on communication and quality of life in some patients. While follow-up care helped identify complications, structured rehabilitation for voice and speech was often lacking, leading to prolonged difficulties with phonation, and swallowing.54 Voice issues refer to problems with sound production, such as hoarseness, breathiness, or reduced vocal strength, whereas speech difficulties involve fluency, and coordination of oral muscles needed for clear communication. Hoarseness and swallowing issues correlated with lower physical component scores, while the unexpected association of hoarseness with improved mental health scores suggests possible adaptive coping mechanisms that warrant further investigation. Despite their benefits, speech-language pathology (SLP) services remain underutilized, delaying recovery and limiting communication abilities.55 Early intervention with SLPs, talking tracheostomy tubes, and phonation strategies could enhance both voice and speech recovery. Additionally, mental health challenges such as anxiety and depression are often overlooked in follow-up care. Expanding structured referrals to SLPs and peer support programs could improve both communication and emotional resilience.56 Standardizing assessments of voice, speech, and swallowing in follow-up care may further optimize long-term recovery and quality of life.
Follow-Up Pathways: Patterns, Timing, and Implications for Tracheostomy Patients
Findings showed variability in follow-up care post-tracheostomy, with 75.5% of patients attending at least one visit and most seen within one month post-discharge, reflecting the importance of timely care to address complications and improve outcomes.47 While otolaryngologists were the most commonly consulted specialists (54.8%), the low rate of endoscopic evaluations (19.3%) raises concerns, as regular assessments are critical for detecting airway and laryngeal complications.18,57 The inconsistencies in follow-up frequency, with 31.3% attending every three months and 13.9% attending only once, suggest gaps in standardized care that could hinder recovery and long-term outcomes.49 High rates of hospital readmissions (60.3%) and one-year mortality (46.5%) among tracheostomized patients further highlight the need for consistent and structured follow-up to reduce healthcare utilization and improve survival.3 Additionally, inadequate continuity of care and provider training,27 reinforce the value of multidisciplinary, patient-centered follow-up systems in addressing these challenges and optimizing recovery.50
Optimizing Long-Term Tracheostomy Care: Challenges, Outcomes, and Interventions
Among participants, 6.8% retained their tracheostomy tubes three years post-discharge, primarily due to tracheal stenosis (33.3%). While not all cases were explicitly linked to iatrogenic injury, prior studies suggest that prolonged intubation and improper tube sizing can contribute to stenosis. Further research is needed to assess the proportion of stenosis cases attributable to iatrogenic factors, including prolonged intubation and improper tracheostomy tube sizing. Optimizing tube selection and follow-up protocols may mitigate these risks. While 13.3% of retention cases were attributed to unknown causes, possible due to gaps in follow-up care.58 Enhanced post-discharge monitoring and standardized decannulation protocols could help address these gaps and optimize long-term outcomes.51,59 The high mortality rate (38.7%) observed among our initial cohort highlights the substantial long-term morbidity associated with tracheostomy. This aligns with prior research demonstrating high one-year mortality rates in critically ill tracheostomy patients.3,60,61 Timing of tube removal significantly differed between those with and without follow-up care, emphasizing the benefits of coordinated aftercare.37 Participants with follow-up were more likely to undergo timely decannulation, whereas earlier, less-monitored removals were more common among those without follow-up. Hospital settings were the predominant site for decannulations, reflecting the need for acute care in managing complex cases.62 Persistent stomas, reported exclusively in follow-up groups, highlight opportunities for innovative interventions such as silicone sealing discs to promote effective closure.63 Cosmetic outcomes also varied widely, with scars ranging from ¼ inch to over 1 inch, impacting patient satisfaction and psychosocial well-being.64 Despite these concerns, plastic surgery assistance was utilized in only 4.9% of cases, suggesting an underutilization of reconstructive options. Given the subjective nature of patient-reported cosmetic outcomes, additional research is needed to validate the impact of follow-up care on stoma appearance and healing. Addressing factors such as wound infection, malnutrition, and prolonged cannulation could enhance recovery and reduce disparities in cosmetic and functional outcomes.65 A multidisciplinary approach to post-decannulation care is essential to improving quality of life for tracheostomy patients.66
Limitations
This study has several limitations that may impact the interpretation and generalizability of its findings. The low response rate (15%) introduces selection bias, as patients with severe illness may have been either over-represented (if more likely to engage with care) or under-represented (if unable to participate due to health limitations), which should be considered when interpreting QoL findings. Additionally, self-reported data on adherence to tracheostomy care routines and complication rates may be subject to recall bias, particularly among patients without follow-up care. Variability in follow-up timing, provider type, and illness severity may have influenced the results despite adjustments in multivariate analyses. The study did not capture the location of patients at the time of survey completion (e.g., home vs. facility-based care), which may have contributed to differences between groups. Furthermore, telephone surveys may have posed challenges for tracheostomy-dependent patients with communication difficulties, affecting response rates and data completeness. The single-center design may limit generalizability to other healthcare settings, and evolving tracheostomy management practices, may have introduced variability in care and outcomes. Despite these limitations, this study provides valuable insights into the impact of follow-up care on tracheostomy management and highlights areas for future research and improvements in post-discharge care.
Conclusion
This longitudinal cohort study highlights the vital role of follow-up care in improving tracheostomy management, adherence to maintenance practices, and long-term outcomes. Patients with follow-up care exhibited better stoma cleaning adherence, timely decannulation, and enhanced cosmetic outcomes, though challenges such as persistent complications, variable care protocols, and disparities in quality of life remained. The findings emphasize the need for standardized, multidisciplinary follow-up systems that incorporate routine evaluations, patient education, and telehealth innovations to address the diverse needs of tracheostomy patients. By refining care models and addressing gaps in provider training, healthcare systems can optimize recovery, reduce complications, and advance evidence-based practices to improve outcomes for this vulnerable population.
Contact Information for Corresponding Author
The Pennsylvania State University
201 Nursing Sciences Building
University Park, PA
Email: vpandian@psu.edu
Phone: 443-655-3482
Funding Statement
Supported by the National Institutes of Health, National Center for Research Resources, Institute for Clinical and Translational Research (TL1RR025007); PI: Daniel Ford.
Acknowledgment for Assistance with Data Collection
We would like to acknowledge Amy Sun, Cari Albers, Emma Lee, Jerusha Naidoo, Johnny Lee, Leila Shirvan, and Sofia Colvin for their valuable assistance with data collection.
Disclosures
Dr. Haut reports research funding (unrelated to this manuscript) from AHRQ, PCORI, NIH/NHLBI. Dr. Hillel: I will disclose that I am a consultant for Airiver, Inc though it has no relation to the content of this manuscript. Dr. Pandian: Funded through AHRQ