1. Introduction
Tracheostomy tubes are life-sustaining medical devices that maintain a secure airway for patients who require prolonged or permanent breathing support. In the United States, 4,800 pediatric tracheostomy procedures are performed annually, typically in children with a mean age of 3 years old.1–4 Globally, 25,000 children require tracheostomies each year, and it is expected that these numbers will grow with improving healthcare quality and access in developing countries, as well as advancements in medical technologies that enable clinicians to keep younger premature babies alive.5 To ensure the safety and quality of life for individuals needing tracheostomy interventions, tracheostomy tubes are employed in various settings including hospitals, homes, schools, and other community areas. As a result, caregivers of tracheostomized pediatric patients range from experienced medical professionals to family members. Despite well-established guidelines for tracheostomy tube care, 20% of tracheostomized pediatric patients experience an emergency complication with their tracheostomy tube that requires immediate caregiver notification and intervention.6,7 Furthermore, infants and children with tracheostomy tubes have difficulty vocalizing to alert caregivers of an emergency due to the presence of a tracheostomy tube, where air bypasses the vocal cords and thus prevents adequate vocalization. Qualitative interviews with caregivers of tracheostomized children also indicate that current training methods and frequency of training are insufficient.8,9 Specifically, nurses, assistant nurses, and emergency medical professionals report a lack of confidence in tracheostomy care skills as a result of low patient volumes, while family caregivers describe anxiety and panic surrounding emergency tracheostomy tube events at home due to insufficient training during patient discharge proceedings.8,9
The leading causes of tracheostomy-related emergencies and fatalities in children are accidental decannulation and obstruction with many of these events occurring outside hospital settings where parents, family members, and at-home nurses serve as primary caregivers.2,10 Studies indicate a 4-6% mortality rate in tracheostomized pediatric patients due to accidental decannulation and obstruction complications.7,10,11 Accidental decannulation transpires when a tracheostomy tube is partially or fully removed from the tracheal stoma. Decannulation can be caused by severe coughing, tracheostomy tube securing tie failure, and pediatric patients pulling on and dislodging tubes. The likelihood of accidental decannulation is increased when patients are not sedated in hospital settings or are highly mobile in at-home environments. For children, unintended decannulation is more common as toddlers improve their mobility and dexterity skills.12,13 Tracheostomy tube obstruction events are a result of mucus occlusions or false passages where the tracheostomy tube is accidentally inserted into the pre-tracheal space. Mucus obstruction complications arise when respiratory tract secretions fully or partially occlude the tracheostomy tube cannula, blocking airflow. When compared to adults, the frequency of mucus obstruction and plugging events is increased in pediatric patients due to smaller tracheostomy tube airway sizes and the absence of inner canula placement.14 False passage, or unintended insertion of the tracheostomy tube into the pre-tracheal space, can happen when a tracheostomy tube needs to be changed during weekly routine or emergency replacements. Furthermore, false passage events are more likely in pediatric patients due to shorter neck anatomy.7,15 Irreversible injury and death are the most severe consequences of accidental decannulation and obstruction events. When a tracheostomy tube is removed via unintended decannulation or obstructed, cardiopulmonary distress and hypoxia begin immediately. If the airway is not re-established within 3-5 minutes by a caregiver, catastrophic injury such as permanent neurological damage or death may likely occur.
Methods to identify tracheostomy complications in patients that require full-time artificial ventilation rely on built-in ventilator sensors to detect most breathing complications, but for individuals who can spontaneously breathe on their own through tracheostomy tubes there are no medical technologies available that can effectively sense a decannulation or obstruction event. Ventilator-independent use of tracheostomy tubes is especially relevant during tracheostomy weaning protocols for pediatric patients which require at least 3 months of no ventilation support.14 To detect emergency tracheostomy tube complications in ventilator-independent patients, caregivers currently rely on visual inspection and pulse oximetry. Visual signs of accidental decannulation and mucus obstruction complications include abnormal chest movements, color change due to lack of oxygenation, and incorrect tracheostomy tube positioning. Furthermore, the tracheal space is not visible during tracheostomy tubes changes so caregivers of tracheostomized patients in at-home environments rely on subsequent indications of breathing to confirm correct tube placement.16 Observation techniques are inefficient due to continuous supervision requirements and many visual indications of tracheostomy complications more difficult to identify in children. Pulse oximetry devices are not specific to tracheostomy tube complications and delay problem identification between 1-3 minutes when the effects of hypoxia may have already started. Infants and children also commonly pull or kick off pulse oximetry monitors, causing false alarms and alarm fatigue for caregivers. A tool that enables real-time and specific tracheostomy tube problem-identification would allow caregivers to spend less time troubleshooting and provide a greater opportunity for airway re-establishment in an emergency event, significantly reducing negative outcomes in pediatric patients.17
Therefore, a custom carbon dioxide sensing system was engineered in the form of a compact, universal tracheostomy tube attachment to monitor for tracheostomy tube decannulation and obstruction emergencies. The sensitized attachment device was utilized to collect breathing waveform data during simulated tracheostomy tube emergency events (e.g., accidental decannulation, improper insertion, and mucus obstruction). To assist with testing new tracheostomy tube technologies for identifying emergency events in ventilator-independent patients, a pediatric tracheal breathing model system was developed to mimic tracheostomy tube respiration patterns. The airway model system consisted of 3D-printed tracheal models and a breathing simulator to create respiration patterns for various pediatric patient age groups (0-3 months, 2-4 years, and 10-12 years old). Results indicated that during incorrect insertion, accidental decannulation, and complete blockage of a tracheostomy tube, exhaled carbon dioxide readings remained at a static baseline while waveforms collected during partial mucus obstruction resulted in decreased signal amplitude relative to unobstructed conditions. Overall, the tracheostomy tube breathing monitor developed in this work will enable caregivers to more quickly identify and specifically detect airway problems in ventilator-independent patients.
2. Methods
A. Custom Carbon Dioxide Monitoring Tracheostomy Tube Attachment
A prototype carbon dioxide sensing tracheostomy tube attachment was engineered with a medical-grade carbon dioxide sensor (USEQGSEAC82180; Kemet, Fort Lauderdale, FL), power management circuitry, a coin cell battery (CR1632), and Bluetooth Low Energy (BLE) microcontroller (Artemis; SparkFun, Boulder, CO). Printed circuit board design software (Eagle; Autodesk, San Fransico, CA) and manufacturing facilities (OSH Park, Lake Oswego, OR) were utilized to fabricate custom circuitry. Calibration of the carbon dioxide sensor was achieved following manufacturers specifications. The microcontroller was programmed to output carbon dioxide sensor readings over a serial communication interface for data collection purposes. Electronics housing prototypes were made with an Original Prusa i3 MK3S+ 3D printer (Prusa Research, Prague, Czech Republic) and polylactic acid filament (eSUN, Shenzhen, China). The size of custom attachment was approximately 2.5 x 2 x 2.5 cm and the weight (including battery) was 8.7 g. To ensure compatibility with existing tracheostomy tubes and accessories, the carbon dioxide monitoring attachment was designed to fit between a standard tracheostomy tube hub (15 mm outer diameter) and a heat moisture exchanger (Figure 1).
B. Pediatric Tracheostomy Model Fabrication
Since pediatric tracheal anatomy and respiration patterns are age-dependent, models were generated for patients aged 0-3 months, 2-4 years, and 10-12 years. Pre-existing 3D digital files representing the upper torso/neck and trachea were utilized to achieve anatomically accurate tracheal models. All virtual prototyping was performed using a Dell Precision 7530 mobile workstation laptop with Onshape (PTC, Boston, MA) and Parasolid (Version 34.1; Siemens AG, Munich, Germany) software. For the 0-3 months, 2-4 years, and 10-12 years age groups, models were generated with an average trachea diameter of 5.25 mm, 7.67 mm, and 12 mm and average neck circumferences of 22.5 cm, 25 cm, and 31.5 cm, respectively (Figure 2).18–20 Tracheas were positioned approximately 3-5.5 mm deep from the outer surface of the anterior portion of the neck depending on the age group.21,22 The trachea was positioned vertically with the assumptions that the top of the larynx was in the same traverse plane as the C5 vertebrae and that the C5 vertebrae was in the same traverse plane where the superior portion of the trapezius muscle meets the neck.23 The printable components of the tracheal models were fabricated with an Original Prusa i3 MK3S+ 3D printer and thermoplastic polyurethane (3D Printing Canada, Hamilton, Ontario). Details on printer settings are available (Supplemental Table 1). To replicate the tracheostomy tube/stomal interface, synthetic skin overlays were fabricated from rubber silicone (Smooth-On Dragon SkinTM 10 NV; Macungie, Pennsylvania) and a fabric mesh. The silicone skin was poured in a mold and left to cure for an excess of 18 hours. Tracheal models were assembled by overlaying the 3D-printed tracheal models with synthetic skin (Figure 3).
C. Breathing Circuit Set-Up for Simulations
To provide pressure-driven respiration function in the tracheal models, an ASL 5000 Breathing Simulator (IngMar Medical, Pittsburgh, PA) was integrated in the model system for simulating breathing patterns in a ventilator-independent patient. Age-dependent breathing parameters (e.g., breathing rate, tidal volume, and resistance) were matched to the neonate, toddler, and pediatric tracheal models (Table 1).24,25 To produce exhaled carbon dioxide in the open-ended model system, an auxiliary gas exchange cylinder (balloon lung) was incorporated along with a gas supply tank. Carbon dioxide delivery was adjusted to match normal, healthy end-tidal carbon dioxide values (35 to 45 mmHg).26 A medical-grade capnograph (EMMA Capnograph; Masimo, Irvine, CA) was used to verify carbon dioxide output levels in the model system. Additionally, a TSI 3076 Aerosol Generator (TSI Incorporated, Shoreview, MN) was used to provide humidity in exhaled breaths of the model system. A humidity sensor (IncuTherm Plus; Incubator Warehouse, Fruitland, ID) was used to confirm moisture levels in the artificial airway. To account for added resistance of downstream simulation components (balloon lung, tubing, etc.), the lung resistance on the ASL 5000 was lowered until tidal volumes matched expected values for each age group.24,25 Output from the breathing simulator, aerosol generator, and carbon dioxide supply was connected to the open-ended tracheal models for emergency tracheostomy tube simulations (Figures 4-5A). An incision was made in the artificial skin of the pediatric tracheal models for subsequent tracheostomy tube insertion (Figure 5B). For the neonate model, a cuffless 2.5 mm inner diameter Bivona neonatal tracheostomy tube (#60SN025) (Smiths Medical, Minneapolis, MN) was used for the simulations. Cuffless Bivona tracheostomy tubes of 3.5 mm inner diameter (#60SP035) and 5.5 mm inner diameter (#60SP055) were implemented in the toddler and pediatric models respectively. Once the tracheostomy tube was in place, the custom carbon dioxide monitoring attachment was coupled to the tracheostomy tube hub (Figure 5C). Since ventilator-independent patients require passive airway humification, a heat moisture exchanger (Ballard 500 pediatric T; Avanos Medical, Knivsta, Sweden) was also added (Figure 5D).
D. Simulation of Tracheostomy Tube Emergency Events
Emergency tracheostomy tube events of incorrect insertion, accidental decannulation, and mucus obstruction were simulated with the ventilator-independent pediatric breathing model system. Incorrect insertion of tracheostomy tube into the pre-tracheal space was emulated by placing the tracheostomy tube cannula between the synthetic skin and underlying 3D printed trachea model. Accidental decannulation of the tracheostomy tube was mimicked by fully removing the tracheostomy tube cannula from the trachea and stoma of the model. Partial and full blockages of tracheostomy tubes were simulated with an artificial mucus (Biochemazone, Leduc, Alberta, CA). Blockage was achieved by gradually adding artificial mucus (0.05 mL increments) directly into the tracheostomy tube. For the 2-4 year-old model, partial blockage was achieved after the addition of 0.15 mL of mucus, accounting for about 40% of the tracheostomy tube volume, and full blockage occurred with the addition of 0.3 mL of mucus. During all simulated tracheostomy emergency scenarios, continuous recordings from the carbon dioxide monitoring attachment were obtained. A wired connection between a computer and the monitoring attachment was utilized for raw data collection. Serial carbon dioxide waveform data was collected with a custom computer program (MATLAB; MathWorks, Natick, MA). A moving average filter spanning 100 data points (1 second worth of data) was applied to smooth carbon dioxide waveform readings for graphing purposes.
3. Results
A. Normal Breathing Measurements
Carbon dioxide recordings were collected during simulations of normal tracheostomy tube respiration in ventilator-independent patients across all tested age groups (Figure 6). As expected, the frequency of carbon dioxide sensor output waveforms corresponded with age-dependent pediatric breathing rate settings (i.e., respiration rate decreases with age). Measured carbon dioxide levels increased with each age group tested during normal breathing simulations with the tracheostomy attachment monitor while standard mainstream capnograph recordings showed exhaled carbon dioxide levels remained constant. Since the attachment monitor directs airflow over the embedded carbon dioxide sensor rather than using a mainstream approach, age-dependent respiration pressures may be impacting carbon dioxide diffusion to the sensing element. Furthermore, the shape of measured carbon dioxide waveforms from the monitor are sharper compared to the rounded rectangular morphology of normal breathing conditions recorded with standard capnographs.
B. Incorrect Insertion Simulation
Simulation during correct placement of the tracheostomy tube into the trachea in a ventilator-independent patient was performed while recording carbon dioxide levels from the monitoring device (Figure 7A & 7C). Prior to insertion of the tracheostomy tube, static ambient carbon dioxide levels were present followed by the generation of exhaled carbon dioxide breathing waveforms with correct placement into the trachea. Carbon dioxide recordings during incorrect tracheostomy tube insertion into the pretracheal space show ambient levels in the absence of exhaled breaths from a ventilator-independent patient (Figure 7B & 7D). The distinct differences between monitor readings with correct and incorrect tracheostomy tube insertion indicated that placement complication detection is feasible with the tracheostomy tube attachment device. Neonatal (0-3 months) and pediatric (10-12 years) recordings during correct and incorrect tracheostomy insertion are available (Supplemental Figure 1 & Supplemental Figure 2).
C. Accidental Decannulation Simulation
During simulated accidental decannulation in a ventilator-independent patient, carbon dioxide level recordings from the monitoring device showed the transition between normal breathing conditions with cyclic breathing patterns to ambient carbon dioxide level (Figure 8). When exhaled carbon dioxide waveforms desist, the tracheostomy tube attachment device could be programmed to quickly trigger an emergency notification to a caregiver. Neonatal (0-3 months) and pediatric (10-12 years) recordings during accidental decannulation in ventilator-independent simulations are available (Supplemental Figure 1 & Supplemental Figure 2).
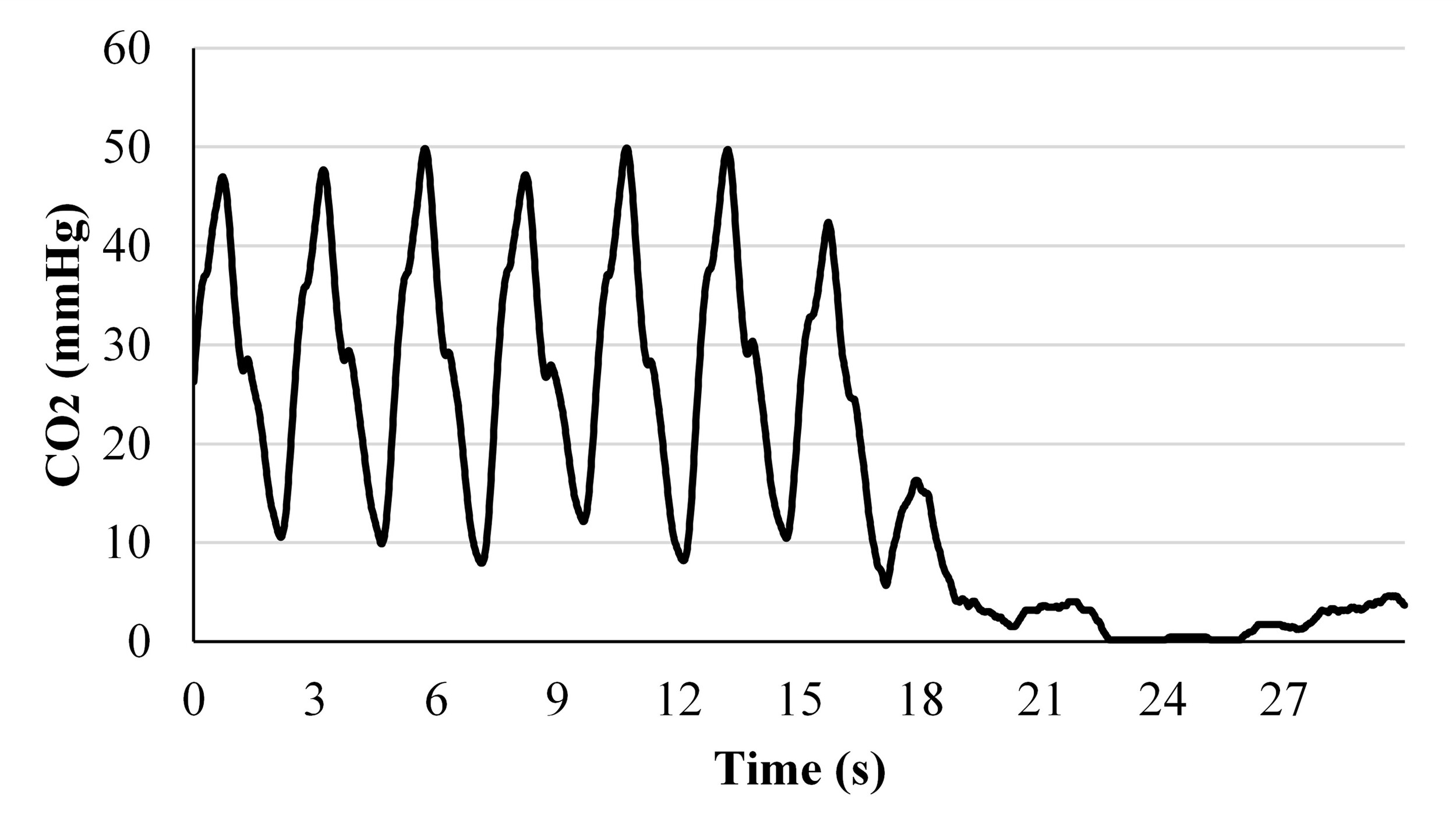
D. Partial and Full Blockage Simulation
Unobstructed respiration through tracheostomy tubes in ventilator-independent simulations indicated periodic exhaled carbon dioxide waveforms (Figure 9A & 9D). With partial mucus blockage of a tracheostomy tube, simulation results showed decreased exhaled carbon dioxide waveform amplitude compared to unobstructed conditions (Figure 9B & 9E). Further testing is required to determine the relationship between exhaled carbon dioxide amplitude alterations and occlusion percentage for indicating when tracheostomy tubes require suctioning or cleaning to remove mucus build-up. During simulated full blockage of a tracheostomy tube, carbon dioxide measurements remained static at ambient levels providing a basis for complication detection (Figure 9C & 9F). Neonatal (0-3 months) and pediatric (10-12 years) recordings during complete mucus obstruction are available (Supplemental Figure 1 & Supplemental Figure 2).
4. Discussion
This study demonstrated proof-of-concept for a tracheostomy tube monitoring system designed to quickly and reliably detect critical airway emergencies such as decannulation and obstruction events for ventilator-independent patients. Using three-dimensional anatomically accurate pediatric trachea models connected to a breathing simulator, we emulated respiration patterns during tracheostomy tube emergency events for ventilator-independent children aged 0-3 months, 2-4 years, and 10-12 years. In addition to assisting with the evaluation of new tracheostomy tube technologies for identifying emergency events in ventilator-independent patients, the tracheal models could also be used for simulation-based medical education allowing caregivers to build and maintain competency and skills in tracheostomy care and complication mitigation.27 Limitations of the study include that the breathing simulator settings were not adjusted to replicate abnormal breathing patterns, such as those found in patients with respiratory complications (e.g., asthma, apnea, hiccupping). Additionally, only cuffless tracheostomy tubes were tested, as ventilator-independent patients typically do not require cuffs unless severe secretion management is necessary. Only one type of heat moisture exchanger was utilized and compatibility with other tracheostomy attachments such as speaking valves were not evaluated. Furthermore, the simulation setup did not account for the impact of patient mobility, vocalization, and swallowing on the function of the monitoring attachment.
In conclusion, this work was necessitated by an accidental decannulation emergency event in a ventilator-independent tracheostomized toddler that was not recognized by a caregiver in time and resulted in mortality. Existing portable, medical-grade carbon dioxide sensing systems are not used to constantly monitor for accidental decannulation or obstruction events in ventilator-independent tracheostomized patients since they are not small and compact enough to be worn continuously (> 5 cm in length and weigh 65 g), have limited battery-life (5-10 hours), are costly (~$1500 not including required consumables), and contain interfaces that are tailored for healthcare professional use. To overcome these constraints, the detection device developed in this study incorporated wireless low-power, carbon dioxide sensing circuitry in the form of a miniaturized tracheostomy tube hub attachment. Functions of the device include: 1) alerting caregivers of accidental decannulation and obstruction, 2) aiding in the confirmation of correct tube placement during emergency and routine tracheostomy tube changes, especially for family members and nurses in at-home settings who often have limited tracheostomy tube care experience, and 3) indicating when tracheostomy tubes require suctioning or cleaning to remove mucus build-up and partial obstructions. The detection tool will also address the limitations of existing monitoring methods by identifying an emergency tracheostomy tube complication faster than standard pulse oximeter techniques, giving caregivers more time to re-establish an airway before permanent injury or death occurs. Overall, this technology will provide a practical solution to preventing injury, death, and recurrent hospitalizations due to pediatric tracheostomy tube complications in ventilator-independent patients.
Future efforts include designing signal analysis software for identifying the presence and type of tracheostomy tube complication from carbon dioxide breathing waveforms and triggering a wireless alert. To provide a simple and accessible interface, remote monitoring in the form of a mobile phone application or baby monitor-like device will be engineered to inform and guide caregivers with visual and audio cues during tracheostomy tube complication mitigation efforts. Since tracheostomy tube attachments increase the risk of accidental decannulation due to additional weight on the tube and attachment projection allowing a patient to grab and pull, device form factor and weight will be minimized as much as possible in accordance with clinical feedback. Furthermore, clinician input indicates rechargeable batteries are desired to improve the safety (i.e., prevent button battery swallowing hazards) and sustainability of the device, therefore the size, weight, and type of battery powering the device needs optimization. Another anticipated challenge includes balancing device cost and infection control methods. For example, the current sensor utilized in the carbon dioxide monitor would require disposal on a single use basis as it is not feasible to wash or sterilize daily. Pricing analysis with existing tracheostomy tube accessories (e.g., disposable, single-use heat-moisture exchangers and washable speaking valves) indicate current products cost approximately $2-4/day, therefore alternative methods and technologies for identifying tracheostomy tube complications with cheap disposable or washable sensing components may be preferred.
Acknowledgements
We would like to thank the MUN MED 3D Lab at the Memorial University of Newfoundland for their expert 3D printed tracheal model work and the Robert E. Fischell Institute for Biomedical Devices at the University of Maryland for their support with respiratory simulations.
Funding
This material is based upon work supported by Innovation Ventures and The Founders Auxiliary Board at Children’s National Hospital (JS).
Conflict of Interest
A provisional patent covering the tracheostomy tube monitor has been submitted to the United States Patent and Trademark Office.

__frontal_cross-sec.jpg)
_and_airway_integration_features_(**b**).jpg)



_and_pretracheal_(**b**)_insertion_of_.jpg)

__partial_(**b**)__and_complete_(**c**)_blo.jpg)

__frontal_cross-sec.jpg)
_and_airway_integration_features_(**b**).jpg)



_and_pretracheal_(**b**)_insertion_of_.jpg)
__partial_(**b**)__and_complete_(**c**)_blo.jpg)