Introduction
Tracheostomy is a common and essential procedure performed in Intensive Care Units (ICUs), with approximately 10-15% of ICU patients requiring this intervention.1 However, the presence of a tracheostomy tube can lead to profound and lasting impairment of communication, significantly impacting the well-being and experiences of patients.2–4 A survey of 115 patients and caregivers from 20 countries revealed challenges with the inability to communicate as the most common tracheostomy care problem.5 The loss of the ability to voice is particularly distressing for ICU patients, as voice serves as a vital means of communication, allowing for self-expression and connection with others.6–8 The absence of voice not only limits patients’ ability to understand and convey information but also hampers their communication abilities and restricts their active participation in their own care, thereby affecting their emotional state and overall satisfaction with their ICU experience.9 Furthermore, the challenges faced by patients with communication difficulties, such as frustration and isolation, have been associated with psychological trauma and increased levels of fear and anger.4,10
While there is existing research on the technical aspects of tracheostomy insertion and decannulation, there is a notable gap in the literature regarding optimal management strategies for patients with tracheostomies during their ICU stay.1 The focus has primarily been on the physiological aspects of care, rather than on addressing the specific communication needs of these patients, such as describing the types of communication devices. Additionally, limited attention has been given to assessing the quality of life; speech intelligibility; time to communication, oral intake, and tracheostomy tube decannulation; and clinical response and tolerance following the use of communication devices.11 There is also a lack of understanding regarding the facilitators and barriers of implementing communication devices to facilitate voice. This lack of research highlights the need for investigations exploring multidisciplinary interventions aimed at enhancing communication for ICU patients with tracheostomy. Such interventions can play a vital role in promoting effective communication, patient-centered care, and improved psychosocial outcomes.
Therefore, we investigated the literature to evaluate the impact of different types of communication devices on quality of life (QOL), speech intelligibility, voice quality, time to significant events, clinical response and tolerance, and healthcare utilization in patients undergoing tracheostomy. Additionally, this review aims to identify and analyze the facilitators and barriers to the implementation of communication devices in this patient population. By synthesizing existing literature, we aim to consolidate existing research to inform best practices, identify gaps for future studies, and explore the effectiveness, implementation challenges, and cost-efficiency of these devices.
Methods
Design
A systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to ensure transparency and reproducibility.12 Our PICO question was, “how do different types of voice-facilitating communication devices affect quality of life (QOL), speech intelligibility, voice quality, time to significant events, clinical response and tolerance, and healthcare utilization in patients undergoing tracheostomy?” In addition, we also explored facilitators and barriers to implementing communication devices.
Eligibility criteria
The inclusion criteria were studies 1) involving adult patients (18 years and above) who have a tracheostomy and are receiving care in the ICU, 2) describing the use of different types of voice-facilitating communication devices, 3) reporting any of the following outcomes: QOL, speech intelligibility, voice quality, time to initial communication device use, oral intake, or tracheostomy tube decannulation, clinical response and tolerance, and healthcare utilization (ICU or hospital length of stay), 4) published in English Language, and 5) and published between 2016 and 2023. 2016 was used as the cut-off since that was when a review of literature on a similar topic was published.13 Exclusion criteria included scholarly materials such as poster abstracts, dissertations, conference proceedings, literature reviews, book chapters, and letters to the editor.
Information sources and Search Strategy
A comprehensive literature search was conducted to identify relevant studies in electronic databases, including PubMed, Embase, Cochrane Library, Scopus, and CINAHL. The search strategy was designed in collaboration with a clinical informationist to ensure thorough coverage of the literature. Both peer-reviewed journals and gray literature sources were considered. The search strategy included terms related to tracheostomy, communication devices, clinical outcomes, patient characteristics, and healthcare utilization. The initial search was conducted in March 2021 and the last search was conducted in June 2023 (Supplemental Online Content).
Selection process
Two independent reviewers screened the identified studies based on the pre-defined inclusion and exclusion criteria. Any disagreements were resolved through discussion or consultation with a third reviewer. The screening and selection processes were facilitated using Covidence, a web-based software platform designed to streamline systematic reviews.14 The reviewers used Covidence to import and screen studies, and any conflicts in study selection were resolved through Covidence’s built-in discussion feature.
Data collection process
The systematic review began with the importation of search results from PubMed, Embase, Cochrane Library, Scopus, and CINAHL into Covidence. Following this, two reviewers independently screened titles and abstracts of retrieved studies against predetermined inclusion and exclusion criteria, with any disparities resolved through discussion or consultation with a third reviewer if necessary. Subsequently, full texts of potentially relevant studies were obtained and assessed independently by two reviewers against the inclusion criteria, with any disagreements resolved through discussion or consultation with a third reviewer as needed.
Data items
The data extraction process involved recording study characteristics (country, design, setting, sample size), patient characteristics (age, sex, race, indication for tracheostomy), tracheostomy tube and communication device characteristics (type, size, device type), clinical outcomes (quality of life, speech intelligibility, voice quality), time to events (days to initial communication device use, oral intake, decannulation), clinical response and tolerance, healthcare utilization (ICU length of stay, hospital length of stay), and facilitators and barriers to implementing speech devices. Each variable was systematically extracted and documented to ensure comprehensive coverage of relevant information for analysis and synthesis. Each data point was reviewed by two reviewers and a consensus was reached by a third reviewer.
Study risk of bias assessment
The Risk Of Bias In Non-randomized Studies - of Interventions (ROBINS-I) tool was used to assess the risk of bias in the included studies.15 Two reviewers independently assessed the risk of bias for each study using the ROBINS-I tool, considering domains such as confounding, selection bias, performance bias, detection bias, attrition bias, and reporting bias. Any discrepancies between reviewers were resolved through discussion or consultation with a third reviewer. The overall risk of bias for each study was then summarized, taking into account the assessments across different domains. This assessment process ensured that the quality and reliability of the included studies were thoroughly evaluated in the systematic review.
Effect measures
The effect measures extracted from the included studies encompassed a wide range of variables related to study characteristics, patient demographics, tracheostomy tube and communication device characteristics, clinical outcomes, time to events, clinical response and tolerance, healthcare utilization, and facilitators and barriers to implementing speech devices. Study characteristics such as country of origin, study design, setting, and sample size were documented to provide context for the findings. Patient characteristics, including age, sex, race, and indication for tracheostomy, were considered potential effect modifiers or confounders. Tracheostomy tube type, size, and communication device type were examined for their influence on clinical outcomes such as quality of life, speech intelligibility, and voice quality. Time to events (defined as from the time of tracheostomy placement) such as days to initial communication device use, oral intake, and decannulation were assessed as indicators of intervention effectiveness and patient recovery. Clinical response and tolerance, healthcare utilization metrics such as ICU and hospital length of stay, and factors affecting the implementation of speech devices were also analyzed to evaluate the overall impact of interventions on patient outcomes and healthcare delivery. Collectively, these effect measures provided a comprehensive understanding of the interventions’ efficacy and their implications for clinical practice and patient care.
Synthesis methods
Descriptive summaries were generated for each extracted variable, summarizing key findings and variations observed across studies after displaying them in tabulated formats. Qualitative synthesis techniques, such as thematic analysis, were employed to identify common themes and patterns across studies, particularly in relation to facilitators and barriers to implementing speech devices. The synthesis process involved iterative discussions among the research team to ensure the accuracy and reliability of the synthesized findings. Finally, the synthesized results were interpreted in the context of the research objectives and existing literature to draw meaningful conclusions and implications for clinical practice.
Reporting bias assessment
Reporting bias was assessed by examining the included studies for indications of selective outcome reporting or publication bias. This involved scrutinizing study protocols, trial registrations, and supplementary materials to compare reported outcomes with those pre-specified in study protocols or registrations. Any discrepancies or concerns regarding reporting bias were discussed among the research team to inform the interpretation of study findings.
Certainty assessment
Certainty assessment of the evidence was conducted using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach.16 This involved evaluating the quality of evidence for each outcome based on study limitations, inconsistency, indirectness, imprecision, and publication bias. The certainty of evidence was rated as high, moderate, low, or very low for each outcome, with rationale provided for each rating. Any disagreements or uncertainties in certainty assessment were resolved through discussion among the research team. The certainty assessment process ensured transparency and rigor in evaluating the strength of evidence and informing recommendations for clinical practice.
Results
Study selection
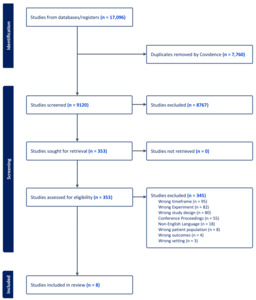
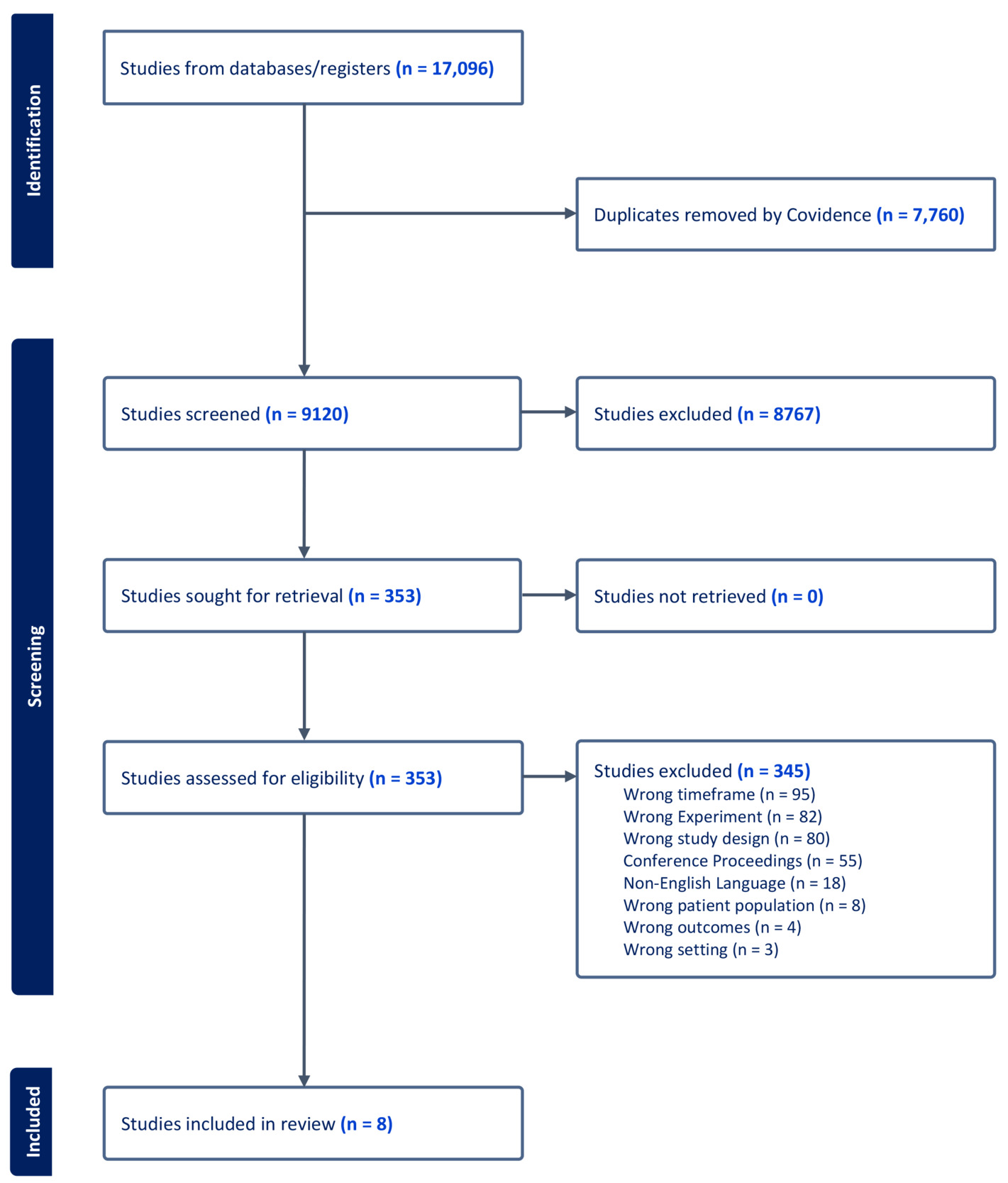
A total of 9,228 studies were identified using the search of databases. After removing 108 duplicates, 9,120 studies underwent screening based on the inclusion and exclusion criteria. Among these, 8,767 studies were deemed irrelevant, leaving 353 for full-text review. Of these, 345 were subsequently excluded (Figure 1). Ultimately, eight eligible studies were included in the review.17–24
Study Characteristics
Studies were conducted in various geographical locations, including Australia (n=5), the United Kingdom (n=2), and the United States (n=1) (Table 1). The study designs included prospective observational studies (n=3), case series (n=2), randomized controlled trials (n = 2), and non-randomized experimental study (n=1), primarily conducted within the Intensive Care Unit (ICU) setting. Sample sizes varied, with the smallest study including 3 participants in one study23 and the largest involving 50 participants.25
Risk of Bias in Studies
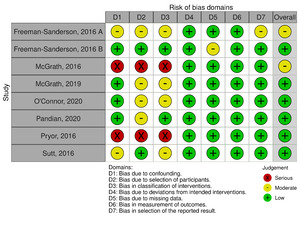
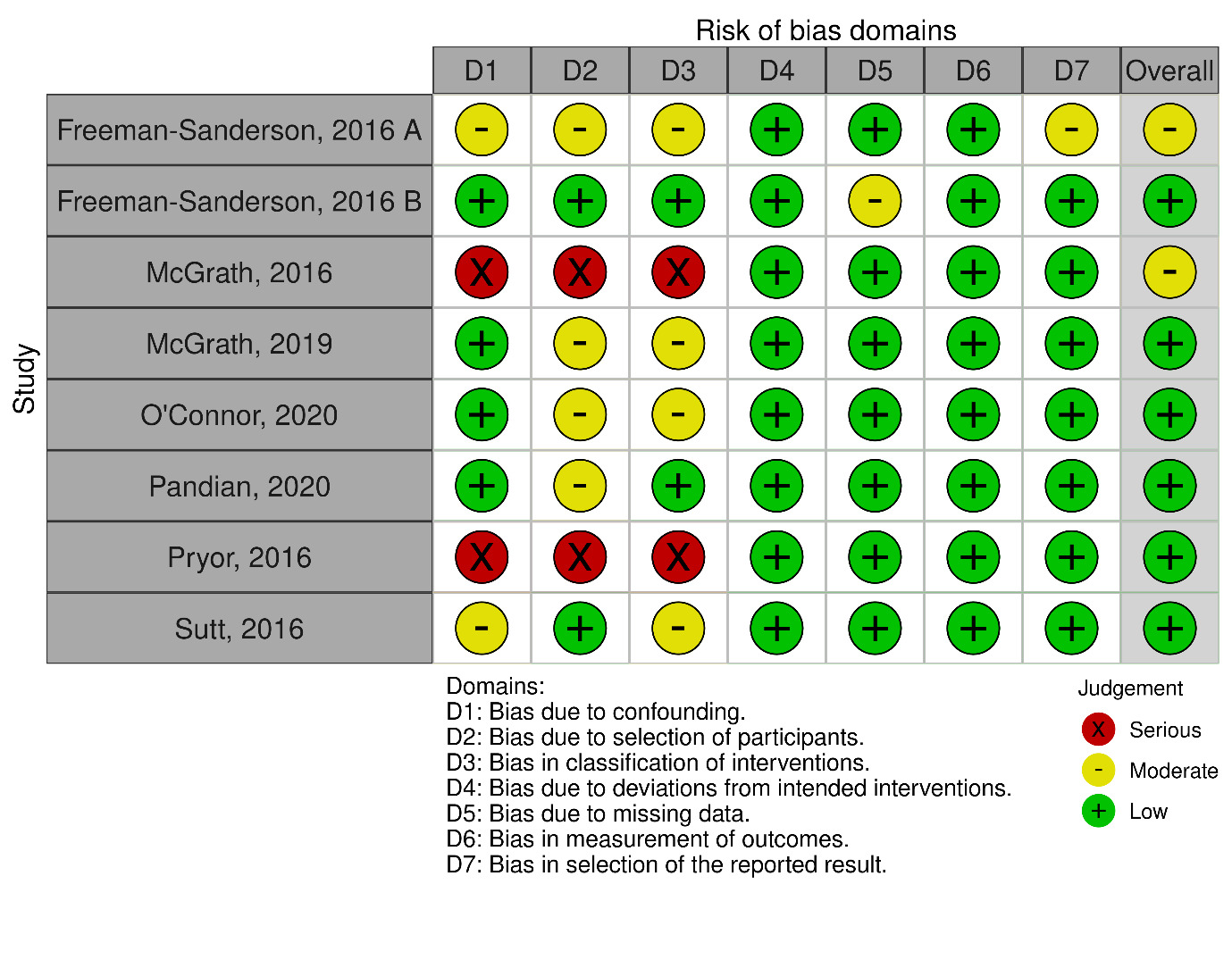
The risk of bias assessment across the included studies revealed varying degrees of methodological quality (Figure 2). Two studies demonstrated a moderate overall risk of bias, primarily due to concerns related to confounding factors and methodological limitations.17,18 One study exhibited a moderate to serious risk of bias, particularly concerning deviations from intended interventions and the measurement of outcomes.19 Four studies had low to moderate overall risk of bias, with minor concerns related to participant selection and measurement of outcomes.20–22,24 One study presented a serious risk of bias, particularly in terms of confounding factors.23 Overall, while most studies had relatively low to moderate risk of bias, careful consideration of potential sources of bias is warranted when interpreting the findings of this systematic review.
Results of Syntheses
Patient characteristics
This review comprised a total of 160 patients for whom age ranged from 36 to 76 years (Table 2), and there were 93 (58%) men and 67 (42%) women. Race was reported only in Pandian et al.,25 study, with 20 (60%) Caucasian, 18 (36%) African American, and one Asian and one Middle Eastern. Tracheostomy was most commonly indicated for chronic respiratory failure (n=68), followed by post-surgical airway management (n=6), aspiration (n=2), chronic hypoxia (n=1), and to maintain airway patency (n=1).
Communication device characteristics
Communication devices included various tracheostomy tubes with speech capabilities or the use of one-way speaking valves (Table 3). There were several brands of tracheostomy tubes used across studies, with Portex and Shiley most prevalent. The tracheostomy tube size 8.0 was commonly used, 24 times across the studies, followed by size 7.0 used 18 times and size 9.0 used 6 times. Tracheostomy tubes with speech capabilities included above-cuff vocalization and fenestrated tubes. The above-cuff vocalization method was used 35 times. McGrath (2019)20 reported the use of Blue Line Ultra Suctionaid (above-cuff vocalization device) as both a tracheostomy tube and communication device. Pandian et al. (2020)25 compared traditional tracheostomy tubes with Blue Line Ultra Suctionaid as an above-cuff vocalization device. Fenestrated tubes such as the Blom® tracheostomy tube system were also utilized in one study.17 Communication devices predominantly consisted of one-way speaking valves (85 instances), including Portex orator and in-line one-way speaking valves (used 35 times), facilitating speech production in tracheostomized patients.
Clinical Outcomes
Quality of Life
Freeman-Sanderson et al. (2016 A)18 reported improvements in various aspects of quality of life following the return of voice (Table 4). Visual analogue self-esteem scale scores increased, indicating enhanced self-esteem associated with regained voice function. In addition, there were notable improvements in feelings of being understood and cheerfulness (p=0.06 and p=0.04, respectively).18 However, no significant difference was observed in EuroQol-5D scores. In contrast, Pandian et al. (2020)25 found significant improvements in voice-related quality of life measures. The experimental group demonstrated a substantial increase in mean voice-related quality of life scores from 39 pre-intervention to 50.2 post-intervention, compared to a modest increase from 48.2 to 48.4 in the control group (p=0.001). Furthermore, specific items related to repetition and outgoing scores showed significant improvements in the experimental group compared to the control group (p=0.001 and p=0.04, respectively).25
Speech Intelligibility and Voice Quality
McGrath (2016)19 reported cases of aphonia and dysphonia, indicating significant voice impairment in some patients (Table 4). Similarly, McGrath (2019)20 assessed voice quality using the GRBAS scale and found median scores indicating moderate grade, roughness, breathiness, asthenia, and strain, suggesting notable voice abnormalities. Pandian et al. (2020)25 observed a significant decrease in speech intelligibility test scores correlating with an increase in SOFA scores, indicating a negative impact of clinical status on speech intelligibility. Pryor (2016)23 evaluated speech intelligibility and voice quality using the GRBAS scale and Voice Handicap Index Score. Patient 1 exhibited a noticeable reduction in speech intelligibility and received voice quality scores of 2, indicating moderate impairment. Patient 2 showed a just noticeable reduction in speech intelligibility and received voice quality scores of 3, indicating more pronounced impairment. Both patients experienced difficulties in vocal projection and strain while speaking, as evidenced by Voice Handicap Index Scores.
Time to Clinical Events
Freeman-Sanderson et al. (2016 B)17 compared the time from tracheostomy placement to initial communication device use between control (Portex orator speaking valve) and experimental groups (In-line one-way speaking valves) and found a significant difference in median days to phonation, with 18 days in the control group compared to 7 days in the experimental group (p=0.001) (Table 4). However, no significant difference was observed in the time to oral intake (p=0.14) or time to decannulation (p=0.38) between the two groups.17 McGrath (2019)20 reported a median of 8 days (IQR: 36) to achieve above cuff vocalization. O’Connor (2020)21 found a median of 11 days (IQR: 6, 14) to achieve the use of a one-way speaking valve.
Clinical Response and Tolerance
Various indicators of clinical response and tolerance were assessed across the included studies, providing insights into the physiological and functional outcomes of tracheostomy management (Table 4). Freeman-Sanderson et al. (2016 B)17 reported incidences of oxygen desaturation <88% and tachypnea >35 breaths/min occurring in both control and experimental groups. Additionally, increased upper respiratory tract secretions were noted in the control group, while the experimental group experienced excessive coughing and hypertension >160 mmHg in one patient each.17 McGrath (2019)20 observed a favorable clinical response, with 66 out of 91 attempts (72.5%) resulting in gained speech. Improvement in unstimulated dry cough and swallow frequency further demonstrated functional improvements following tracheostomy management. O’Connor (2020)21 reported a median duration of 10.5 hours (Range: 5.5 – 17) for using a one-way speaking valve. Pryor (2016)23 assessed the ability to sustain phonation in two patients, with mean durations ranging from 30-60 minutes to 2-3 hours at a time, indicating varying degrees of tolerance to vocalization tasks. Sutt (2016)24 observed improvements in end-expiratory lung impedance with one-way speaking valve use, along with decreases in end-tidal carbon dioxide and respiratory rate, while oxygen saturation and heart rate remained stable, suggesting physiological adaptation to the use of communication devices.
Healthcare Utilization
The assessment of healthcare utilization parameters provided insights into the impact of tracheostomy management on hospital resources (Table 4). Freeman-Sanderson et al. (2016 B)17 found no significant difference in Intensive Care Unit (ICU) length of stay between the experimental and control groups (p=0.69). However, there was a difference in hospital length of stay, with the experimental group staying 14 days longer on average than the control group, although this difference was not statistically significant (p=0.42). McGrath (2019)20 reported a median ICU length of stay of 28 days (IQR: 36), indicating a prolonged duration of critical care management for tracheostomized patients. O’Connor (2020)21 observed a median ICU length of stay of 37 days (IQR: 28, 49), suggesting a substantial utilization of intensive care resources in this patient population. Pandian (2020)25 compared healthcare utilization between control and experimental groups, finding a median ICU length of stay of 29 days in the control group and 49 days in the experimental group. Similarly, hospital length of stay was longer in the experimental group compared to the control group, with medians of 60 days and 35 days, respectively.25
Facilitators and Barriers to Implementing Speech Devices
Early intervention by speech pathology during mechanical ventilation emerged as a facilitator in expediting the restoration of speech, as highlighted in Freeman-Sanderson’s study (2016 B).17 This early, targeted intervention significantly hastened the return of voice, thereby enhancing communication outcomes within the ICU setting. Moreover, improvements in communication and quality of life were noted to coincide with the restoration of voice, emphasizing the interplay between effective intervention and positive patient outcomes, as demonstrated in Freeman-Sanderson’s work (2016 A).18 Patient perceptions and comfort also played a crucial role in facilitating successful speech restoration, with patient-reported preferences for speech cannula placement indicating the importance of addressing individual comfort and communication needs, as underscored by Pryor (2016).23 Additionally, maintaining laryngeal function was identified as essential for successful artificial communication voice restoration, highlighting the need for cooperation and intact laryngeal function in facilitating positive outcomes, as elucidated by McGrath (2016).19 Early signs of tolerance to communication devices further facilitated their use, with patients demonstrating tolerance after an initial transition period, as observed in O’Connor’s study (2020).21
Despite these facilitators, several barriers were identified that impeded successful speech restoration and communication outcomes. Variability in access to speech-language pathology (SLP) services within ICU settings presented a significant barrier, with some centers unable to provide adequate support, as noted by Freeman-Sanderson (2016 A).18 Moreover, psychological factors such as low affect were observed to hinder recovery by reducing patient engagement and participation, indicating the complex interplay between psychological states and communication outcomes, as highlighted by Freeman-Sanderson (2016 A).18 Upper airway secretions were identified as a physical barrier that interfered with voice quality, potentially obstructing the gas flow line and impacting communication effectiveness, as outlined by McGrath (2016).19 Intolerance factors, including issues related to airway patency and respiratory muscle weakness, further posed challenges to the successful implementation of communication devices, as detailed by O’Connor (2020).21 Additionally, poor speech cannula placement and drug-induced sedation were identified as specific barriers that hindered the effective use of communication devices, underscoring the importance of addressing technical and pharmacological challenges in tracheostomy management, as elucidated by Pryor (2016) and O’Connor (2020), respectively.21,23
Certainty of Evidence
The certainty of evidence varied across studies: Freeman-Sanderson, 2016 A,18 McGrath, 2016,19 and Pryor, 201623 provided low-quality evidence, while McGrath, 2019,20 O’Connor, 2020,21 and Sutt, 201624 presented moderate-quality evidence (Figure 3). Pandian, 2020 and Freeman-Sanderson, 2016 B offered high-quality evidence.17,25 These assessments inform confidence levels in the effectiveness of tracheostomy management strategies.
Discussion
This systematic review found that different types of communication devices significantly impact the quality of life (QOL), speech intelligibility, and voice quality in patients undergoing tracheostomy. Studies showed improvements in self-esteem, cheerfulness, and voice-related QOL with the use of these devices, though results varied across specific metrics like speech intelligibility and voice quality. Time to significant events, such as phonation and decannulation, was reduced in some instances, indicating potential for faster recovery processes. Clinical response and tolerance varied, with some patients experiencing minimal side effects. Healthcare utilization outcomes, such as ICU and hospital length of stay, showed mixed results, with no significant differences in some studies. Facilitators for implementing communication devices included early intervention and improvements in communication and QOL, while barriers involved variability in speech-language pathology services, upper airway secretions, and intolerance factors.
Patient Characteristics
Patient characteristics, such as age, sex, race, and medical indications for tracheostomy, varied across the studies, reflecting the diverse nature of tracheostomized populations, similar to other studies that describe the characteristics of patients with a tracheostomy.2 Age has been identified as a predictor of quality of life and mortality among patients with a tracheostomy.26,27 Female sex has been identified as a predictor of reporting communication issues related to tracheostomy decision-making in patients receiving prolonged mechanical ventilation.28
Communication device characteristics
The communication devices employed in the included studies encompassed different tracheostomy tube types (Portex cuffed non-fenestrated tubes, Blue Line Ultra Suction tracheostomy tubes, and Shiley Evac (Blom TTS fenestrated) tubes) and speaking valves (Passy-Muir speaking valves with ventilators, Passy-Muir speaking valves on tracheostomy collar (off the ventilator)). Alternative communication methods included communication boards or iPads. Findings highlight the availability of multiple options for speech restoration in the ICU, allowing healthcare professionals to tailor interventions to individual patient needs. Prioritizing communication in the ICU has resulted in earlier introduction of communication devices,21 earlier return to voice,17 and the ability for patients to tolerate the communication device for an extended period.23 In 2019, Zaga’s literature review highlighted similar communication modalities mentioned above, in addition to the use of an electrolarynx and a combination of multiple modalities.13 In 2019, Karlsen’s literature review identified the need for future studies to compare the various communication aids against each other to strengthen the evidence on already existing communication aids.
Quality of Life, Speech Intelligibility, and Voice Quality
The role of communication emerges as a salient determinant22,25,29 of quality-of-life for patients navigating the complexities of tracheostomy-related care. Findings from our review revealed notable improvements in quality of life, speech intelligibility, and voice quality following tracheostomy management interventions. Emotional factors, such as anxiety and frustration, were identified as barriers to successful speech restoration. The patient’s inability to communicate with nurses and family members, even if non-verbal language was used, resulted in increased negative emotions such as helplessness, frustration, embarrassment, and depression.30–32 Additionally, Zaga’s literature review (2019) further stated that patients experience psychological distress and feelings of powerlessness during their ICU admission when their communication is significantly compromised.13 Studies in our review reported significant enhancements in various domains of quality of life, particularly in areas related to communication and voice-related activities, indicating the profound impact of interventions aimed at restoring voice function. However, challenges such as speech intelligibility impairments and voice abnormalities were also observed, underscoring the multifaceted nature of communication difficulties in tracheostomized patients. Factors affecting voice quality included upper airway secretions and blockage of the gas flow line above the cuff, which may affect the clarity and audibility of speech.19
Clinical responses and tolerance
Findings suggest a generally positive adaptation to communication devices. Instances of oxygen desaturation and tachypnea were present in both experimental and control groups, with some patients experiencing increased secretions, coughing, or hypertension. However, a majority of attempts to gain speech were successful, with noticeable improvements in cough and swallow functions. Usage duration of one-way speaking valves varied, reflecting different patient tolerances for vocalization tasks. Physiologically, one-way speaking valves contributed to improved lung impedance and reduced carbon dioxide levels, without adversely affecting oxygen saturation and heart rate, underscoring their potential for enhancing respiratory function following tracheostomy.24 Factors such as airway patency, tracheomalacia, vocal cord paralysis, inelastic lung recoil, and difficulty exhaling around the tracheostomy tube can contribute to intolerance and impact the effectiveness of speaking devices.21
Healthcare utilization
Data on healthcare utilization suggests that the implementation of tracheostomy management strategies did not significantly alter the length of ICU stay when comparing experimental (In-line one-way speaking valve) and control (Portex orator speaking valve) groups.17 Additionally, while there was an observed difference in hospital length of stay, it was not statistically significant.17 McGrath 2019 and O’Connor 2020 reported median ICU stays of 28 and 37 days, respectively, but did not report on hospital length of stay. Pandian 2020’s findings suggest that the experimental group (Blueline Ultra Suctionaid) had a notably longer ICU and hospital stay compared to the control (one-way speaking valve) group. These findings highlight a potential increase in healthcare resource use associated with certain tracheostomy interventions, though the implications of this increase require further investigation to understand the underlying causes and to determine the clinical significance.
Facilitators and Barriers to Using Communication Devices
Facilitators such as early intervention by speech pathology, patient perceptions, and comfort, and laryngeal function maintenance emerged as key factors contributing to successful tracheostomy management. However, barriers including variability in access to speech-language pathology services, psychological factors, upper airway secretions interference, and intolerance factors posed challenges to effective communication and patient care. In addition to the barriers indicated in the articles included in our literature review, Pandian et al (2022) identified reduced access to care or supplies as a barrier to promoting effective oxygenation and communication, especially in the context of the recent COVID-19 pandemic.8 Another potential barrier mentioned in articles outside of this literature review is the great physical and mental effort needed to communicate with a tracheostomy in place.30,33 Addressing these barriers through comprehensive care strategies tailored to individual patient needs is essential to optimize outcomes and enhance patient experiences in tracheostomy management. Overall, this review provides valuable insights into the complexities of tracheostomy management and underscores the importance of multidisciplinary approaches and patient-centered care in optimizing outcomes for tracheostomized patients.
Implications for Interprofessional Teams
Speech-language pathologists (SLPs), physicians, nurses, and respiratory therapists are critical for optimizing communication for tracheostomy patients. The review findings emphasize the need for collaborative care planning to select and implement the most appropriate communication devices based on individual patient needs, which can improve QOL and expedite recovery. Providers should consult SLPs more frequently as they play an important role in assessing speech intelligibility and voice quality, which will guide the team to tailor interventions appropriately, providing early targeted therapy to restore communication abilities effectively. Providers with prescriptive authority need to pay attention to the overall clinical response, monitor tolerance to devices, and adjust medical treatment as needed. Bedside nurses providing ongoing care should know the differences between tracheostomy tube types and communication devices to be able to monitor patient vitals in response to the devices, and be the first to identify signs of intolerance or complications.34 Nurses should also educate patients and their families about communication strategies and assistive devices to facilitate effective communication during the recovery process.35,36 Respiratory therapists should collaborate with SLPs and nurses to make ventilator setting changes to promote use of communication devices.
Collaborations among interprofessional teams can also aid in addressing affective factors and psychological well-being,3,37–40 which are crucial for successful speech restoration.41–44 Providing emotional support to patients and addressing anxiety and frustration can engage patients in speech restoration efforts.45
Furthermore, findings underscore the necessity of regular, open communication among team members to address and navigate the identified barriers, such as variability in SLP services and the impact of upper airway secretions. The team must be attuned to the facilitators of successful device use, such as patient comfort and perceptions, which require the concerted efforts of the entire care team to support and reinforce. Collectively, these professionals must also be cognizant of healthcare utilization metrics, working together to streamline processes that could lead to shorter lengths of stay and better resource management without compromising patient outcomes. Interprofessional education and protocols regarding the use of communication devices may help standardize care, mitigate variability in services, and ensure a patient-centered approach to tracheostomy management, however, this requires further investigation.
Implications for Health Policy
The findings have several implications for health policy in the context of ICU settings. Access to speech-language pathology services should be emphasized, addressing staffing needs and potential barriers to access.46,47 Interprofessional collaboration and effective communication among healthcare professionals should be promoted.48–51 Health policy should support outcome measurement and quality improvement initiatives, prioritize patient-centered care and informed decision-making, and allocate resources for research and evidence generation to drive evidence-based practices and enhance the delivery of speech restoration interventions in the ICU.2,52
Limitations
Limitations of this study should be acknowledged to provide context for interpreting the findings and understanding the potential implications for future research and clinical practice. First, despite efforts to conduct a comprehensive literature search across multiple databases and sources, some relevant studies may have been missed, leading to potential selection bias. Additionally, the inclusion criteria limited the review to studies published in English from 2016 onwards, potentially excluding relevant non-English studies and earlier publications that could contribute valuable insights. Moreover, the variability in study designs and outcome measures across the included studies introduces heterogeneity that may affect the generalizability of the findings. The small sample sizes and varying clinical contexts of the included studies further limit the statistical power and applicability of the results to broader patient populations. Finally, the complexity of tracheostomy management and the multitude of factors influencing clinical outcomes necessitate cautious interpretation of the findings, recognizing the need for further well-designed studies to elucidate the optimal approaches to tracheostomy care and communication device selection in ICU settings.
Conclusion
This systematic review elucidates the substantial benefits of communication devices in enhancing quality of life, speech intelligibility, and voice quality for patients with tracheostomy. The data demonstrates notable improvements in self-esteem and cheerfulness, contributing to increased voice-related quality of life. Although variability was observed, there is a suggestion of accelerated progress toward critical recovery milestones such as phonation and decannulation. Clinical responses and tolerance to these devices varied among patients, with a generally low incidence of side effects. In terms of healthcare utilization, the findings were mixed, indicating that while some patients may benefit from shorter stays, the impact of communication devices on overall hospital and ICU length of stay is inconclusive. Effective implementation of these devices is facilitated by early intervention and the observed enhancements in communication, yet is challenged by the availability of speech-language pathology services and physiological barriers. The review highlights the multifaceted implications of communication devices in tracheostomy care, reinforcing the necessity for individualized patient assessments to optimize outcomes.
Corresponding Author
Vinciya Pandian, PhD, MBA, MSN, ACNP-BC, FCCM, FAANP, FAAN, FFNMRCSI
Associate Professor and Assistant Dean, Immersive Learning and Digital Innovation, Johns Hopkins School of Nursing, Baltimore, Maryland, United States
443-655-3482
Funding
Vinciya Pandian receives support from NIH (R01NR017433-01A1) for the study exploring symptoms of laryngeal injury post extubation in the intensive care units.
Conflict of Interest / Financial Disclosures
Other authors report nothing to disclose.